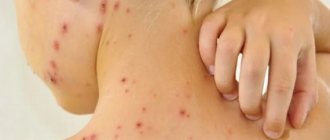
Causes of non-healing wounds
The immediate cause of non-healing wounds is insufficient activity of restoration processes in the body, slow tissue regeneration. Moreover, the conditions under which regeneration slows down can be varied.
Among the general factors (i.e., affecting the state of the body as a whole), the following can be identified:
• weakened immunity due to concomitant disease or chronic immunodeficiency state;
• chronic intoxication of the body;
• metabolic disease;
• diabetes; this condition can be considered a severe form of metabolic disorder, namely glucose metabolism. Disturbances in the normal absorption of glucose lead to increased tissue fragility and a slowdown in the regeneration process;
• circulatory disorders - both general and local. The cause may be atherosclerosis, deformation of vascular walls, and diseases of the cardiovascular system. Local circulatory disorders can be caused by prolonged compression of tissues and pinching of small blood vessels (an example of a chronic wound against the background of constant compression of tissues - bedsores);
• hypo- or avitaminosis of vitamins A, C, K and group B, which play an important role in the process of tissue regeneration;
• lack of calcium or zinc in the body;
• exhaustion – nutritional (due to insufficient or malnutrition) or senile;
• presence of cancer.
A specific factor in the appearance of chronic wounds is poor circulation in the legs, most often due to varicose veins. Against the background of impaired blood circulation, trophic ulcers develop on the legs (usually in the shins) - difficult-to-heal tissue defects.
Local factors affecting the wound itself and the area around it include:
• infection;
• the presence of necrotic tissue in the wound (they increase the risk of suppuration and, accordingly, the duration of healing);
• presence of foreign bodies in the wound;
• the presence of bleeding in the wound (also increases the risk of infection);
• repeated damage to an unhealed wound.
The risk of a wound becoming chronic increases if, in addition to the skin, other tissues are damaged - subcutaneous tissue, nerves, muscles, tendons, bones.
Main types of chronic wounds
- Diabetic foot syndrome (DFS) is a set of anatomical and functional changes that develop against the background of diabetes mellitus, increasing the risk of injury and infection of the soft tissues of the foot. According to the World Health Organization, the risk of developing DDS in diabetics with 5-10 years of experience is about 15%. For diabetics with more than 15 years of experience, it increases to 90%.
- Prevention: protecting feet from injury, comprehensive foot and nail care, wearing shoes for diabetics.
- Trophic ulcers are complications of venous insufficiency caused by changes due to thrombophlebitis or deep vein varicose veins. Poor circulation and blood supply to the legs leads to venous congestion, and this leads to the formation of poorly healing ulcers on the ankles or legs.
- Symptoms are swelling, impaired skin pigmentation (redness or pallor), eczema, a feeling that the legs are bursting from pressure (more often in the evening).
- Prevention: timely treatment of the underlying disease, wearing elastic tights, treating the skin with antiseptic and healing agents.
Symptoms of non-healing wounds
•constant pain;
•converging edges of the wound;
•constant bleeding;
• “stuck” wound at one of the stages of healing for more than three weeks;
•often – unpleasant odor;
•conventional treatments do not help or help little.
These signs indicate that the regeneration processes in tissues are disrupted for one reason or another. For the treatment of chronic wounds, local treatment is not enough; comprehensive measures are required.
To achieve progress in healing, it is necessary to provide conditions for restoring the normal rate of regeneration. They largely depend on the cause of the chronic wound, but there are recommendations that are common to all situations.
The body needs to provide:
•balanced nutrition - in particular, the diet should be enriched with vitamins A and B, which stimulate tissue regeneration, and vitamin C, which promotes the production of collagen - a substance involved in the formation of connective tissue that closes the wound in the first stages;
•normal sleep mode – during sleep the regeneration process occurs more actively;
•protecting the wound and the area around it from external influences, such as rubbing, squeezing, etc.;
•wound ventilation – without air circulation, the wound will quickly fester.
More specific recommendations depend on the origin and nature of the wound.
Treatment of non-healing wounds taking into account their cause
When starting to treat injuries of this kind, it is necessary to understand the cause of their occurrence. Further actions will depend on this.
1. First of all, it is necessary to examine the wound for foreign bodies, necrotic tissue, and bleeding and, if necessary, clean it. Please note that it is strongly recommended not to remove foreign bodies yourself! Such attempts may cause additional tissue damage and increase the risk of infection. You need to seek help from a doctor.
2. If local blood circulation is impaired, it is necessary to eliminate the source of pressure on the tissue (change the position of the patient’s body with bedsores, wear looser clothes/shoes for “diabetic” wounds).
3. When treating trophic ulcers on the legs, it is necessary to use compression stockings or bandages - they will help “support” the blood vessels and normalize blood circulation, as well as “pull” the edges of the wound towards each other for faster healing.
4. In case of exhaustion and vitamin deficiencies, it is necessary to provide a normal balanced diet in order to provide the body with the necessary substances for tissue regeneration and the production of collagen - a substance that is actively involved in the early stages of the healing process and helps tighten the edges of the wound.
5.If you have diabetes (of any type), you need to monitor your blood glucose levels.
After creating optimal conditions for wound healing, you can begin to treat and treat it.
Products and dressings for the treatment of chronic wounds
The course of therapy is selected by the attending physician; you should not self-medicate - this can lead to a worsening of the situation.
Gels and solutions for rinsing and cleansing
- Gels fill subcutaneous pockets and cavities, providing an antimicrobial effect (Prontosan gel), cleanse necrotic masses (Askina gel, Hydrosorb gel), form an optimal environment for healing (Purilon gel), and work as an anti-inflammatory antimicrobial agent (Emalan collagen hydrogel).
- Prontosan sterile solution is suitable for preventing infections, cleaning and moisturizing wounds, helping in tissue regeneration and suppressing pathogenic microflora.
Principles (stages) of treatment
Actions when treating a wound depend not only on the conditions and causes of its appearance, but also on the stage at which the wound process has stopped in it.
The first stage of the wound process is acute. During it, vascular changes occur caused by a reaction to damage, and the process of blood clotting is actively underway. If the wound is infected, pus is formed and toxic substances are simultaneously absorbed into the tissue.
At this stage, it is necessary, first of all, to clean the wound - remove dead tissue from it, remove foreign bodies (with the help of a doctor), drain it - remove pus and exudate from the wound. It is also necessary to prevent infection from entering the wound. It is best to wash the affected area with saline solution, as caustic disinfectant liquids can harm surrounding healthy tissue.
The second stage of the wound process is granulation. At this stage, a thin layer of connective tissue begins to form on top of the wound. The edges of the wound begin to tighten, and it closes itself. The thinnest capillaries are formed in the connective tissue layer, providing nutrition to regenerating tissues. Often chronic wounds stop at this phase, and the healing process does not proceed further; This is most often what happens with trophic ulcers.
At the granulation stage, it is important, firstly, to protect the newly formed tissue from mechanical damage, secondly, to stimulate further regeneration, and thirdly, to prevent the development of the inflammatory process - despite the fact that the wound is closed, the risk of infection and inflammation remains. To stimulate tissue regeneration, methyluracil ointment is used (you can use a ready-made Voskopran dressing with methyluracil ointment); to protect the surface of the wound, atraumatic dressings should be used that do not stick to the wound surface and do not injure it.
We should not forget about maintaining a maintenance diet and sleep schedule - this will help speed up the healing process.
The wound process is a complex set of reactions that develop in the body in response to tissue damage. From the point of view of general pathology, the healing of any wound is inflammation, which successively goes through the stages of alteration (damage), exudation (the release of the liquid part of the blood and inflammatory cells outside the vascular bed) and proliferation (restoration of lost tissue through scar formation) [8]. However, it turned out to be clinically convenient to divide the healing phases somewhat differently. In the domestic literature, the more accepted classification of the Department of Wounds and Wound Infections of the Institute of Surgery named after. A.V. Vishnevsky, dividing the course of the wound process into three main phases.
I - inflammation phase;
includes
vascular reactions
(vasoconstriction followed by vasodilation),
exudation
with the release of plasma proteins, migration and release of blood cells into the damaged area, fibrin loss with delimitation of the damaged area, edema and infiltration of surrounding tissues.
Subsequently, fibrin undergoes fibrinolysis and the wound is cleansed
of necrotic tissue and microorganisms with the participation of leukocytes and their enzymes. It begins immediately after injury and, in the absence of complications, lasts an average of 4-5 days.
Some researchers propose to distinguish alteration phases in the first stage of the wound process,
including primary destruction and accompanying biochemical changes, and
exudation and secondary destruction,
during which the dead tissue is rejected and the wound is cleansed.
II - phase of regeneration (reparation),
or
the formation and maturation of granulation tissue,
is characterized by the migration of fibroblasts, their formation of collagen and ground substance, new formation of blood vessels and the development of granulation tissue at the site of the tissue defect. Gradually, exudation and edema decrease, and granulation tissue fills the entire defect. This phase begins on the 1st day after injury and lasts on average 2-4 weeks. Its duration depends on the size of the wound defect and the morphological structure of the damaged tissue.
III - phase of scar formation, reorganization and epithelization,
it cannot be clearly separated in time from phase II. Epithelization begins from the edges of the wound simultaneously with the formation of granulation tissue. Immediately after the formation of a scar, its restructuring begins: elastic fibers and a new fibrous network are formed, and the water content in the scar tissue decreases. The epithelization process is regulated by the action of epidermal cheilon, which is a contact inhibitor of proliferation. The most important condition for normal wound healing is, on the one hand, strict synchronization of the epithelization process, and on the other, the maturation of granulation tissue. The balance between the maturation and resorption of granulations and scar tissue underlies the phenomenon of wound contraction - a uniform concentric contraction of the edges and walls of the wound.
In phases II and III of healing, wound contraction, as a rule, is combined with intensive epithelization, which indicates the normal course of the wound process. Depending on the morphological structure of the tissues, the process lasts from several months to a year [3, 22, 24].
The above classification quite accurately reflects the main stages of the wound process and makes it possible to determine pathogenetically targeted treatment according to the phase of wound healing.
Foreign authors distinguish the stages of healing in approximately the same way:
— Inflammatory phase (stage of inflammation);
— Proliferative (also called “Reconstruction”) (reparation stage);
— Maturation and remodeling phase (remodeling stage) [24].
Taking into account modern views, one cannot ignore the active participation in the early stages of the wound process of uncontrolled formation of free radicals in cells as a result of a “respiratory explosion” in polymorphonuclear leukocytes. In these cells, powerful biooxidants are formed - reactive oxygen species (ROS), which have pronounced cytotoxic activity towards bacteria and eukaryotes [25]. At the same time, ROS are the initiator of free radical reactions (FRR) of lipid peroxidation (LPO), which currently play the role of the fundamental molecular basis of various pathological processes. In addition to the activation of phagocytes, among the mechanisms that ensure an increase in the production of ROS that can trigger CRR, the following should be highlighted:
— disruption of transport in the mitochondrial respiratory chain;
— intensification of the synthesis and oxidation of catecholamines;
— activation of the xanthine-xanthine oxidase system [23].
The main substrate of CPP are unsaturated fatty acids, which are part of the phospholipids of cell membranes, membranes of subcellular organelles and blood plasma lipoproteins. It has been shown that under physiological conditions, CPP products are involved in the regulation of biomembrane permeability and the rate of cell proliferation. In the case of excessive accumulation of fatty acid hydroperoxides, inhibition of protein synthesis, blockade of macrophage functions (chemotactic and enzymatic activity), disintegration of cell membranes, inactivation of thiol enzymes, etc. are observed. Free radicals can change the conformation of proteins, increase the sensitivity of substrates to proteolysis, damage the DNA molecule up to its fragmentation [6].
As part of the general adaptation syndrome, the regulation of SRR LPO in the wound is carried out by turning on several parallel mechanisms. Despite the significant diversity, they can be grouped into two types: initiation of the formation of free radicals and elimination (interception) of lipid peroxidation products. Normally, the processes of initiation and elimination are controlled by the action of a balanced endogenous system of hydrophilic and hydrophobic free radical scavengers (antioxidants). However, during injury, hyperproduction of free radicals is observed (especially in the early period of inflammation) with a simultaneous decrease in the activity of endogenous antioxidants, which shifts the balance towards accelerating the SRR of LPO. The essence of the manifestation of the degradative effect of ROS under conditions of their hyperproduction in the wound is the formation of highly reactive hydroxyl radicals OH-, which, under conditions of decreased activity of the antioxidant defense system, trigger secondary SRR of lipid peroxidation of cell membranes not only in the area of the wound defect, but also in perifocal cells zones. As a result, there is a delay in the development of the phases of the wound process that follow inflammation. Of course, such a development of events indicates a breakdown of the general adaptation syndrome and can be regarded as a sign of an unfavorable course of the wound process. The introduction of certain substances into the wound defect causes the production of ROS. Thus, the use of a Cu-ascorbate mixture, xenobiotics, and zymosan caused the induction of the production of hydroxyl radicals in the tissues of the wound defect. An increase in ROS content always led to a delay in wound healing, determined morphologically. It should be noted that the processes of activation and elimination of lipid peroxidation products repeatedly replace each other at various stages of the wound process. Activation of LPO predominates at the stages of inflammation and granulation formation, inhibition - during the period of wound cleansing and epithelization. The period of rumen reorganization is characterized by a balance of pro- and antioxidant systems. This fact demonstrates the cyclical nature of the wound process and gives grounds to consider healing from the point of view of biorhythmology.
The course of the wound process is influenced by various general and local factors. The course of the wound process is worsened by the presence of multiresistant associative microflora, a high degree of microbial contamination, the presence of foreign bodies, and impaired outflow of wound fluid. The course of the wound process is slowed down by deterioration of regional arterial and venous circulation, anemia, malnutrition and decreased immunity, the presence of concomitant diseases such as diabetes mellitus and collagenosis, as well as the use of glucocorticoids and cytostatics [3].
In modern clinical surgery, it is generally accepted that any accidental wound is bacterially contaminated, or primarily infected. The term “bacterially contaminated wound” should be understood as a condition of the wound in which general and local defense mechanisms are able to suppress microorganisms that have entered the wound and there are no clinical signs of an infectious process in the wound. Consequently, the mere presence of microorganisms in a wound (even pathogenic ones, not to mention the group of opportunistic ones) does not make the development of infection in the wound mandatory. According to modern concepts, lysis of necrotic tissue in the first phase of the wound process occurs not only under the influence of wound exudate enzymes. Microflora plays a decisive role in this complex process. It has been established that in the initial period of the inflammatory reaction, microorganisms initiate the migration of leukocytes to the source of infection, and at a later date, by enzymatic breakdown of the necrotic substrate, they remove from the wound everything that was not removed mechanically. Thus, it appears that from a biological point of view, the presence of microorganisms in the wound is appropriate. For this reason, I.V. Davydovsky [17] believed that microflora, being a passive “product of a wound,” is not harmful and the fight against it, carried out since the time of Lister, is meaningless. Until the end of the 19th century, when antiseptic and aseptic methods of wound management were proposed, healing through suppuration was perceived as normal. In addition, microbial contamination of a wound is not always accompanied by classic symptoms of wound infection. For example, a large number of bacteria are present on the surface of chronic wounds that often have no signs of inflammation, such as trophic ulcers or bedsores. However, such colonization, which does not manifest itself as inflammation, can quickly develop into life-threatening septicemia if there is inadequate or no treatment.
Infection in a wound develops when there is an imbalance between microorganisms contaminating the wound and the defenses of the macroorganism, which is manifested by clinical symptoms of inflammation. The significance of bacterial contamination of wounds for the victim is determined by many factors: the absolute and relative number of microbial bodies, their pathogenicity, and the ability of the body’s defenses to fight a possible infection. From a clinical point of view, the leading factor determining the possibility of a bacterially contaminated wound becoming infected is the functional state of the damaged tissue. The development of infection is most likely in extensive bacterially contaminated wounds containing a large amount of non-viable or damaged tissue, which serves as an excellent breeding ground for bacteria. For a comparative assessment of the ability of various microorganisms to cause an infectious process, a number of qualitative indicators have been developed that characterize the degree of their aggression. These include: pathogenicity, virulence and toxicity.
To implement an infectious process in a wound, microorganisms must have certain quantitative (the number of pathogens entering the wound) and qualitative (invasiveness factors) characteristics, which are inversely related.
For purulent wounds of various origins, it is characteristic that among the representatives of the wound microflora at all stages of examination of patients, according to various authors, staphylococci predominate, which are isolated both in monoculture and in various associations. According to most literature sources, there is a fairly high percentage of isolation of gram-negative microorganisms, especially Pseudomonas aeruginosa, the frequency of detection of which increases significantly during patients’ hospital stay [4, 7]. In recent years, under the influence of various factors, primarily the powerful selective action of antibiotics, significant changes have occurred in the etiology of wound infections. The dependence of the microflora of wounds on their origin is clearly visible. So, for example, if in acute purulent diseases staphylococcus in monoculture is detected in 69.5% of cases, then in post-traumatic purulent wounds, chronic purulent diseases of the skin and soft tissues, as well as in purulent wounds and developed sepsis, several pathogenic microorganisms are sown in 31. 5, 48.8, 55.6% of cases, respectively. The rest are representatives of the family Enterobacteriaceae
in monoculture.
Long-term studies of the qualitative composition of the microflora of various wounds indicate a stable predominance of staphylococci (S. aureus, S. epidermidis)
and non-fermenting gram-negative bacteria.
At the same time, in the case of generalization of the infectious process in patients staying in hospital for a long time, the species composition of the microflora isolated from wounds is much wider compared to microorganisms isolated from blood cultures. In recent years, fungi have begun to emerge from wounds much more often (9.9%), which is apparently due to insufficient attention to this problem and the lack of reliable prevention. Obligate non-spore-forming anaerobic microorganisms also play a significant role in the etiology of wound infection, among which the most common are Bacteroides
spp.,
Fusobacterium, Peptococcus
spp.,
Peptostreptococcus
spp.,
F. nucleatum, P. melaninogenicus.
The proportion of pure non-clostridial and mixed aerobic-anaerobic microflora also depends on the location and origin of the purulent wound [4].
With the development of suppuration, even an initially normal wound process initially slows down and stops, then begins to develop in an undesirable direction [3]. Bacterially contaminated wounds become infected and then purulent with changes in color, odor and a sharp increase in exudate production. It is known that the threshold number of pathogens at which a wound becomes infected is 105 per 1 g of tissue. However, numerous studies of this indicator in various wounds indicate that the quantitative microbial landscape of the wound surface can change almost daily. The activity of bacterial enzymes (toxins) underlies the slowing, stopping, and often reversing process of wound healing. Inflammation in response to microbial aggression can occur at any stage of healing, returning the wound process to stage I again [3].
Modern methods of treating purulent wounds
The main method of treatment, which allows you to quickly clean the wound from necrotic tissue, fully drain it and create conditions for healing, is surgical treatment of the wound, washing it with antiseptic solutions (if possible with a pulsating jet), draining the wound and its early surgical closure [13]. In the domestic literature, the volume of surgical treatment has been interpreted differently to date. More often, after sufficient dissection in the area of the purulent focus with the opening of all purulent leaks under visual control, surgeons excise only clearly necrotic tissue, followed by drainage of the wound in various ways [20]. Other surgeons are in favor of a more radical (maximal) removal of the entire purulent-necrotic sequestration [14]. However, in practice, these principles of surgical treatment of a purulent wound are not always followed. So, M.I. Kuzin et al. [21] believe that complete surgical debridement of the wound is possible in 70-80% of patients, and only in 35% of cases can primary sutures be applied after it. The impossibility and inappropriateness of surgical wound closure determine the need for local treatment. We emphasize that surgical treatment, local and drug therapy for a purulent wound are competing or interchangeable methods. They can only be considered as complementary components of complex treatment of a purulent wound.
In addition to surgical cleansing of the wound, in modern literature there are four more methods: autolytic, physical (mechanical), enzymatic and the so-called biosurgical. The choice of method depends on many factors, such as the size, location, type of wound, the required efficiency and selectivity of necrectomy, the level of pain, the amount of exudate, the risk of infection and the cost of treatment. In most observations, it makes sense to sequentially or combined use of several methods of wound cleansing.
Autolytic cleansing
is observed to one degree or another in all wounds. It is a natural and highly selective process carried out by endogenous proteases that break down necrotic tissue. The main sources of proteases are neutrophils that produce elastase, collagenase, myeloperoxidase, hyaluronic acid and lysosomal enzymes.
Autolytic cleansing may not provide rapid cleansing and healing of the wound, but the use of occlusive dressings can significantly speed up this process by maintaining a moist environment and allowing the drainage of exudate. This promotes painless cleansing of dead tissue and the formation of healthy granulations. Autolysis can lead to the formation of significant amounts of exudate. Typically, autolytic cleansing is achieved by using occlusive dressings to create a moist wound environment. The increase in antibiotic resistance of microorganisms has revived some interest in the use of honey for the treatment of wounds and trophic ulcers. The antimicrobial effect of honey provides rapid autolytic cleansing and deodorization of wounds; in addition, it has anti-inflammatory properties and stimulates the immune response. Although the exact mechanism of action is unclear, A. Tonks et al. [30] revealed a significant decrease in the activity of free radical oxidation products (p<0.001); the production of TNFα was also significantly increased (p<0.001) with applications of different types of honey. From a practical point of view, autolytic wound cleansing is the simplest method, but it usually requires too much time to completely clear necrotic tissue.
Physical (mechanical) cleansing
Wound wounding is a non-selective method of removing necrotic tissue and debris through the use of mechanical force. The method is easy to perform and gives a faster effect than autolytic or enzymatic necrectomy. However, the non-selectivity of the method can lead to significant damage to healthy granulations, often creating significant discomfort for the patient due to pain. Despite these disadvantages, several types of mechanical wound cleansing are used. Wet-dry dressings are the simplest method of mechanical cleansing. A wet gauze dressing is placed on the wound bed and gradually dries, absorbing necrotic tissue and detritus. Together with the removable bandage, necrotic tissues that have been absorbed into it are mechanically removed from the wound [26, 27].
Today, in the treatment of purulent wounds, a huge arsenal of technical means is used that provide one or another physical
impact on the wound. This is the treatment of wounds with a pulsating jet. Treatment of the wound with a stream of water or antiseptic under high or low pressure leads to the washing out of bacteria, foreign bodies and necrotic tissue from the wound. However, if the pressure is too high, there is a risk of bacteria and debris penetrating deeper into the wound or damaging viable tissue. Using a pulsating jet can be very effective in softening and removing detritus, exudate, and bacteria from a wound. The positive effect of using a CO2 laser has been convincingly proven. In the treatment of purulent wounds, the technique of using low and medium frequency ultrasound has proven itself well. Due to the fact that ultrasound propagates differently in living and non-viable tissues and is reflected at their interface, it accelerates the processes of rejection of necrotic tissues. At the same time, many authors point to the damaging effect of ultrasound on healthy tissues [13]. Wound evacuation is a non-invasive method of mechanical cleansing by creating negative pressure in a sealed system over the wound bed. It is used in the treatment of chronic wounds and can reduce the amount of exudate and sloughing necrotic masses, reduce tissue swelling, increase the speed of peripheral blood flow, improve tissue oxygenation, stimulate angiogenesis and granulation growth [16]. In recent years, the physical and biostimulating activity of NO-containing plasmadynamic gas flow in relation to wounds has been actively studied.
Enzymatic wound cleansing
is a highly selective method that uses natural proteolytic enzymes produced by the pharmaceutical industry specifically for wound treatment [9-11]. The introduced proteases act in the wound together with endogenous enzymes of the body [15]. Basically, enzyme preparations are produced on the basis of bacterial collagenase, papain/urea, trypsin, fibrinolysin/DNase, a combination of streptokinase - streptodornase and subtilisin. Only the first three products are widely represented on the market. In different countries, one or two of these drugs are traditionally used, depending on the availability of raw materials. Enzyme preparations are used in combination with other biologically active drugs [5].
Biosurgery.
The use of fly larvae for the cleansing and healing of wounds has been known since the first publications in 1931. This method requires the use of sterile larvae that digest the sloughing necrotic masses from the wound without damaging the surrounding normal tissue. In a study by K. Mumcuoglu et al. [29], complete wound clearance by larvae was achieved in 38 (88%) of 43 patients with chronic venous ulcers and pressure ulcers. In 5 of these cases, amputation of the limb due to ulcers was initially planned. Sherman, observing a group of 103 patients with pressure ulcers, noted that when using larvae, complete wound cleansing was achieved in 80% of observations, compared to 48% when using wet-dry dressings. The exact mechanism of wound cleansing and healing with the help of larvae is not completely clear. There are suggestions that they are able to absorb and kill bacteria, give a bacteriostatic effect by increasing the pH of the wound, secrete proteolytic enzymes and increase the supply of oxygen to tissues. However, despite the optimistic data mentioned above, some patients report pain during treatment with larvae. Also, one cannot ignore the aesthetic and psychological aspects of using fly larvae.
Along with local methods of treating a purulent wound, general drug therapy should not be underestimated. These are primarily antibacterial drugs [4]. The widespread use of antibiotics, the effectiveness of which was not questioned for the first decades after their invention, already caused a number of problems by the 70s of the last century. The mutagenic effect of antibiotics on microorganisms has led to a change in the etiological and biological nature of purulent processes. As numerous studies show, the structure of causative agents of purulent complications of wounds has changed [4, 7]. Along with traditional gram-positive and gram-negative aerobic microorganisms, a significant proportion falls on anaerobic microorganisms and fungi. The association of aerobes and anaerobes in purulent post-traumatic wounds, bedsores, and in patients with “diabetic foot” occurs in 98.8% of cases. From 80 to 100% of isolated strains are insensitive to penicillin, cephalothin, cefazolin, tetracycline, kanamycin, and gentamicin. It must be emphasized that we are talking about the systemic use of antibiotics. Local use of antibiotics is usually not recommended (especially by foreign authors), since it can lead to allergic reactions or the emergence of antibiotic-resistant strains of bacteria. It is advisable to directly inject into the wound only some specially developed antibiotics: polymyxin M, neomycin, gramicidin [21, 28].
Currently, against the backdrop of a reassessment of the place of antibiotics, interest in antiseptics has revived. The latter are chemical substances (regardless of the source and composition) that have antimicrobial properties and are used for application to damaged and intact skin, mucous membranes, cavities and wounds for the purpose of treating and preventing the development of local infectious lesions and sepsis. It is argued that for wounds of mild and moderate severity, one can limit oneself to the local use of antiseptics and antibiotics. Only when the wound process is complicated by the development of acquired immunodeficiency, local use of antiseptics should be combined with parenteral administration of antibiotics that do not have cross-resistance with locally applied antiseptics. Among antiseptics, the most commonly used solutions are: hydrogen peroxide (3%), potassium permanganate (0.1-0.5%), boric acid (1-3%), dioxidine (1%), chlorhexidine (0.02%). Aqueous solutions of hydrogen peroxide and potassium permanganate, traditionally used to treat purulent wounds, do not significantly affect the microbial flora; their antiseptic effect is limited to the wound surface and does not extend deep into the tissues where microorganisms nest. The use of a hydrogen peroxide solution meets the requirement of gentle mechanical treatment rather than disinfection [27]. Currently, a large number of antiseptic drugs are known that have good bactericidal properties [4]. However, most of them contain various irritating substances, such as mercury, iodine, polyvinylpyrrolidone, which cause an allergic reaction in a number of patients, which limits their use. Among the modern means, the drug Lavasept (an aqueous solution of biguanide polyhexanide) deserves attention, showing high activity against bacteria and fungi, including staphylococci, enterococci, Pseudomonas aeruginosa and Escherichia coli, and does not inhibit the formation of granulation tissue in the wound. Currently used antiseptics generally have a limited spectrum of action. But the doctor cannot always determine in a timely manner the type of pathogen and its spectrum of sensitivity, and recently purulent processes are caused by associated microorganisms. The process of formation of resistance of microorganisms equally applies not only to the group of antibiotics, but also to traditional antiseptics. For example, solutions of furatsilin, rivanol, boric acid (3%) almost completely lost their antimicrobial activity against the main causative agents of surgical infections. Sensitivity of hospital strains of S. aureus, E. coli, B. coli
to antiseptics and fat-based ointments does not exceed 1-5% [4].
At least 25% of all patients who present with wounds require only initial treatment with dressings [12].
A bandage is one or more dressings applied to damaged or intact human skin in order to create conditions for his recovery. This definition emphasizes the therapeutic effect of the dressing on the entire body, and not just on the wound.
Requirements for dressings at different stages of the wound process
It is important to emphasize that the requirements for dressings clearly change at different stages of the wound process. For example, fresh wounds, until they are completely covered with granulation, are able to absorb toxins, bacteria, and tissue breakdown products. Wounds covered with granulations have virtually no absorption capacity. In this regard, in the first phase of the wound process, all therapeutic drugs must have high osmotic activity in order to ensure intensive outflow of exudate from the depths of the wound into the dressing, antibacterial effect on infectious agents, rejection and melting of necrotic tissue, and evacuation of wound contents. Thus, the role of the dressing in the first phase of the wound process is as follows:
— removal of excess exudate;
— irreversible elimination of bacteria, toxins, wound detritus, dirt, foreign bodies;
— stimulation of rehydration of necrotic areas and acceleration of necrolysis;
— absorption of exudate by the structure of the dressing material and its strong retention [18].
At each dressing, the wound is cleaned of pus and sequestration, necrotic tissue is excised and washed with antiseptics. Currently, ozonated saline solutions are also used to wash wounds. To accelerate necrolysis, proteolytic enzymes, ultrasonic cavitation, vacuum treatment of the wound, pulsating jet treatment and other already mentioned physical methods of influence are used [19].
In phase II of the wound process, along with the suppression of microorganisms remaining in a small number or newly emerging hospital strains due to violation of asepsis and antisepsis at the time of dressings, the drug must provide optimal conditions for the growth of granulations [4]. The role of the dressing in the second phase of the wound process:
— maintaining and regulating a moist environment in the wound;
— ensuring adequate conditioning of the wound;
— protection of granulation tissue from mechanical damage during dressing;
— reliable protection against secondary infection.
During the phase of scar formation, reorganization and epithelization, when choosing a particular drug, the main thing is to ensure optimal conditions for regeneration. To do this, it is necessary to create a hypoadhesive layer of the dressing, which supports hydration and gas exchange in the granulating tissue and protects it from damage during the next dressing. When the wound dries out, a crust forms, slowing down epithelization, and with excess moisture, epithelial cells die. It follows that the dressings should still keep the wound in a moderately moist state and protect from damage, therefore, at this stage of the wound process, sea buckthorn and rosehip oils, aerosols, ointments containing glucocorticosteroid hormones and an antibacterial component are indicated. Fat-based ointments are also used to prevent injury to the young epidermis, as well as various knitted wipes that have a moderate dehydrating effect, which has a beneficial effect on the growth of granulations and epithelization [1]. The role of the dressing in the third phase of the wound process:
- keeping the wound moderately moist;
— protection of the epithelium and the developing scar from mechanical damage during dressing;
— stimulation of regeneration [2].
Choosing a dressing depending on the type of wound
As the technology for making dressings becomes more complex, they become more and more specific. At the current stage of development, no one dressing can be equally suitable for treating all types of wounds, just as several different dressings cannot treat the same wound equally effectively throughout all stages of its healing. Thus, the choice of a dressing for treating a wound, taking into account its characteristics, the stage of the wound process, and the presence of complications, should be based on an understanding of the mechanism of histogenesis and the regulation of reparative regeneration processes.
Dressings for the treatment of non-healing wounds
When treating wounds of any nature, including chronic ones, the correct selection of dressing material plays an important role.
Dressing material for non-healing wounds should be:
•hygroscopic;
•"breathable";
•elastic and flexible – the bandage will have to be worn for a long time, so it should not interfere with movements.
Biotekfarm company offers a wide selection of dressings for the management of wounds of any complexity and at any stage. So, for wounds in which there are fragments of necrotic tissue or hemorrhage, a Parapran dressing with chymotrypsin is suitable - the drug included in the dressing helps to break down necrotic tissue, helping to cleanse the wound.
To moisturize “dry” chronic wounds (bedsores, trophic ulcers) and accelerate their healing, it is recommended to use the “GelePran” dressing with miramistin or colloidal silver. This soft transparent dressing, consisting of 70% water, moisturizes the surface of the wound, and the medicine it contains (miramistin or silver) disinfects and promotes regeneration.
For the treatment of infected wounds, bactericidal dressings Voskopran with Povidone-iodine ointment, antimicrobial dressings Voskopran with Dioxidin ointment, and anti-inflammatory dressings Voskopran with Levomekol ointment are suitable. Polipran dressings with Dioxidin are excellent for preventing infection of wounds at the granulation stage.
The Chitopran dressing based on chitosan nanofibers will help stimulate regeneration. It provides optimal conditions for healing - sufficient humidity, breathability and protection from damage, and promotes the rapid growth of its own cells. It is worth paying attention to the fact that this bandage does not need to be removed - it resorbs on its own as the wound heals.
The infection must be suppressed
To combat infection in a wound, it is best to use ACTICOAT and ACTICOAT7 dressings. These are mesh absorbent dressings that allow unimpeded drainage of exudate from the wound and fight infection. These dressings contain silver. The antibacterial activity of silver has been known for many years and its effectiveness is beyond doubt. The activity of silver is not affected by the presence of blood and pus in the wound. All causative agents of wound infections, including antibiotic-resistant bacteria, are not resistant to silver. Silver has a universal, wide spectrum of action, including against mixed microflora containing various bacteria, fungi, and yeast. The use of dressings with silver leads to rapid suppression of the inflammatory reaction in the wound, and at the same time the amount of discharged exudate decreases.
It is important that the silver in ACTICOAT and ACTICOAT7 dressings begins to be released immediately as soon as the dressing is applied to the wound, and quickly, within the first 30 minutes, the necessary concentration of silver is achieved in the wound to fight infection, and this concentration of silver is maintained at a constant level throughout long-term - up to 3 days when using ACTICOAT dressings and up to 7 days when using ACTICOAT7 dressings, thereby ensuring long-term antibacterial activity of the dressings, so the ACTICOAT dressing can be on the wound for up to 3 days, and the ACTICOAT7 dressing up to 7 days, with external absorbent dressings (gauze or Allevyn sponge) must be changed daily.
Use an acticoate dressing to effectively fight infection.
Wound healing time and consequences
Healing a chronic wound is a long and complex process that can take from 1 to 3 months, depending on the depth and size of the wound. It should be borne in mind that epithelization of chronic wounds is very slow - no more than 1 cm per month. After complete epithelialization, the “new” skin increases its strength for another 6 months.
Recommendations for caring for the problem area are quite simple:
•protect the wound from compression and mechanical damage;
•change the bandage regularly (depending on the stage of healing and type of bandage);
•at the granulation stage - use dressings that accelerate healing.
Excess exudate
If there are no crusts or necrosis in the wound, then the technique of local negative pressure can be used for active outflow of exudate. For these purposes, the PICO wound treatment device is used, which creates a constant outflow of purulent exudate from the wound, which is accompanied by a decrease in tissue swelling, a decrease in the number of bacteria in the wound, as a result, the inflammatory reaction subsides, and the growth of new tissues begins, the vessels that fill the wound begin the epithelium grows, the edges of the wound gradually come closer together and the wound heals. When using this device, there is no need to carry out dressings daily. It is enough to change the dressings on average once every three days.
For passive drainage of exudate from the wound, absorbent dressings of your choice are used:
- Carbonet. This multi-layer dressing perfectly absorbs viscous purulent exudate, and, thanks to a layer of activated carbon, absorbs the odor that often accompanies purulent wounds. Since Carbonet does not contain antimicrobial components, dressings on purulent wounds must be changed daily.
- NEOFIX polyurethane sponge dressings with silver - NEOFIX FibroSorb AG and NEOFIX FibroSorb AG Sacrum. Silver in dressings provides antimicrobial effect.
Sponge dressings absorb liquid exudate and retain it in their structure even under pressure, for example, under compression bandages or underwear (stockings, stockings, tights), which are one of the main attributes of the treatment of trophic venous ulcers. NEOFIX FibroSorb AG sponge dressing
without adhesive edge is indicated for wounds where the skin around which is damaged.
Such a bandage requires additional fixation with a bandage or adhesive roll bandages NEOFIX ROLL, or OpSite Flexifix, always only around the perimeter, without covering its central part with adhesive patches. This is necessary to prevent maceration of the skin and wound. NEOFIX FibroSorb AG Sacrum bandage
with a sticky edge, specially shaped for fixation in the sacrum area, while the skin around the wound should be dry and healthy, without any disturbances. The bandage protects the wound from the ingress of urine, feces, as well as from the penetration of external liquids during hygiene procedures.
When using NEOFIX FibroSorb AG and NEOFIX FibroSorb AG Sacrum dressings for wound treatment, there is no need to change dressings daily. It is recommended to change the dressing once every 2-3 days, depending on the degree of filling of the dressing with exudate.