Hydrogen peroxide will help remove local stains, greasy stains on collars and cuffs, yellowness and grayness. To bleach laundry, you will need pharmacy 3% peroxide, which is used in combination with plain water at 30–35°C. Things are soaked in a warm solution. Baking soda, lemon juice or ammonia will help enhance the effectiveness of whitening. The product is not suitable for washing colored items and clothes with a pattern.
Hydrogen peroxide is a colorless liquid that is an excellent solvent. You can purchase the drug at any pharmacy; the cost of one 100 ml bottle is about 15 rubles.
Useful properties of hydrogen peroxide
This drug has a lot of useful properties. It is used not only in classical medicine to disinfect wounds, but also has many other uses.
Hydrogen peroxide
It is used for:
- treatment of ear diseases and diseases of the oral cavity;
- eliminating fungal infections and mold;
- removing blemishes from the skin;
- hair lightening;
- teeth whitening.
This is only a small part of the list. But most often the product is used in the household. Hydrogen peroxide is great for whitening clothes. In addition, this is an affordable and quite economical way to return things to their former snow-whiteness.
Why is boiling necessary?
In hot water, the temperature of which is 100 °C, traces of chocolate, juice, milk and cottage cheese, pumpkin or carrot puree are washed off, and light-colored clothes are washed well. Mothers who care about their baby’s health resort to boiling laundry to:
- Protect your child from infection.
- Return yellowed items to their snow-white hue.
- Destroy germs, dust mites.
The ancient method of washing clothes is time consuming but environmentally friendly and is used for bleaching.
Although some automatic machines have a boiling function, water in this mode does not heat up to 100 ° C, so not all pathogenic microorganisms die.
Guide to Bleaching with Peroxide
Peroxide can be used in its pure form.
First you need to heat the water
To bleach white items with hydrogen peroxide, use only a 3% solution. More concentrated preparations corrode the skin.
What to do:
- Heat 5 liters of water to 30–35°C. If you have a large amount of clothing to bleach, increase the amount of liquid.
- Dissolve 3 teaspoons of hydrogen peroxide in it.
- Immerse items that need bleaching in this solution.
- Leave for 20–25 minutes.
- Stir the water periodically and turn the products over to get better results.
- After the allotted time has passed, take out the items, wash and rinse. Leave to dry.
If there are stains from coffee, tea, fruits, berries, or blood on clothes, pour the product onto the stained area, leave for a few minutes and wash as usual.
Advice! When washing by hand, it is better to use rubber gloves.
Various boiling methods
Detergents and heat treatment options are selected depending on the type and color of the fabric and the degree of contamination.
Recipes for boiling white textiles
Doctors advise boiling things for babies and infants. In such children, synthetic products irritate the skin. People suffering from allergies have to avoid household chemicals.
Washing powder and bleach
To restore freshness to yellowed textiles and wash away stains and dirt on clothes, boil the items for 30–45 minutes, dissolving half a glass of bleach and powder in water. For boiling, you need to use only an enamel basin or pan, otherwise there will be traces of rust on light-colored laundry, or it will take on a dark shade.
Washing powder and hydrogen peroxide
If a stain from coffee, fruit juice, or vegetable puree appears on white clothes, you can clean the stain and refresh your T-shirt, undershirt or blouse using a blister of hydroperite tablets. Boil the products for about 30 minutes.
Chlorine bleach and table salt
If things are very dirty, first soak them for several hours and then prepare a solution for boiling. Natural fabrics become softer and restore color when boiled in a composition that is prepared by mixing 500 ml of bleach with chlorine and 2 cups of ordinary salt in water. In order not to disturb the structure of the fibers or damage the fabric, you need to boil things for no more than half an hour.
Vegetable oil bleach
By boiling, you can even deal with old stains, which are very noticeable and spoil the appearance of light-colored clothing. To remove dirt by boiling, use 250 ml of sunflower oil, 200 g of washing powder and the same amount of bleach. It is recommended to boil things for a maximum of 5 minutes.
With boric acid
To wash stains on T-shirts, T-shirts, blouses, the items are soaked for half an hour in warm water, into which 7 tablespoons of a liquid solution of boric acid are poured, boiled for about 30 minutes. The items acquire a snow-white hue, and fungal spores die.
For any color
Clothing of different colors, both light and dark cotton fabrics, can be washed by boiling.
Laundry soap with soda
To make stains easier to remove, old stains are soaked for a couple of hours before boiling. Forty grams of laundry soap are ground on a grater and sent into the water. To soften it, add 4 tablespoons of baking soda and 3 ash. Boil things in the prepared solution for half an hour, light denim clothes for 25 minutes.
Salt with water
To give freshness to the linen and improve the appearance of a product that has been soaked and washed by hand, warm water is poured into an enamel pan, into which 250 g of soda, a glass of salt and a little powder are poured.
For baby clothes
The bedding, rompers and undershirts of the baby are first washed with soap without dyes and fragrances and placed in a basin, which is half filled with water. Pour 1 cap of gel for washing baby clothes into the container or use powder that does not contain phosphates, anionic surfactants, fragrances, add grated laundry soap. Boil things for 15 minutes, bleach for an hour.
For work clothes
It is difficult to wash overalls, jackets and robes stained with grease, covered with fuel oil stains, both in the machine and by hand, but this can be done by boiling. Pour a bucket of water into a basin, add 2 pieces of crushed laundry soap, add one and a half cups of dry silicate glue, add 300 g of soda ash. The container with overalls is placed on the fire and boiled. Petroleum products are removed more easily if a couple of tablespoons of kerosene are poured into the solution.
After an hour and a half, things are taken out of the basin, placed in soapy water and boiled again for 30–45 minutes, rinsed several times, starting with hot liquid and ending with cold.
Products that, when interacting with them, increase the whitening effect
The effect of peroxide can be enhanced significantly by combining the product with other whitening components.
Peroxide + soda
Baking soda has an average whitening effect, but in combination with peroxide it works no worse than professional bleaches. But you need to know exactly how to bleach linen with hydrogen peroxide and soda: proportions, holding time, so as not to spoil bed or kitchen towels.
Hydrogen peroxide and soda
To restore whiteness to the most problematic areas (collar, cuffs):
- Mix 10 ml of peroxide and 1 teaspoon of soda.
- Leave for a quarter of an hour on areas that require treatment.
- Then rinse, wash, rinse.
Important! Do not leave the baking soda paste on the product for more than 5 minutes.
General information
Do not confuse hydrogen peroxide solution with regular hydrogen peroxide, which can be harmful. 3% hydrogen peroxide should never be taken. Can be diluted in water.
Many sources talk about the benefits and positive aspects after taking it, while keeping silent about possible risks and dangers.
Hydrogen peroxide exists in 2 forms - liquid and tablet form.
- Perhydrol (3% peroxide in liquid form);
- Hydroperite (35% peroxide in tablet form).
1 tablet contains 1 tbsp. l. liquid solution.
Due to its liquid form, perhydryl is widespread and easy to use in medicine. Hydroperite is usually used independently at home.
Peroxide + ammonia
To get rid of grayness:
- Soak the product in 5 liters of heated water with 1 tbsp. spoon of peroxide and 1 tbsp. spoon of ammonia.
- Wait 30–45 minutes.
- Wash your clothes.
A mixture of peroxide and ammonia
10 liters of hot water, 2 tbsp. spoons of peroxide and 1 tbsp. spoon of ammonia. Dip the curtain into this solution, do not hold it for more than 5 minutes, remove it, and rinse thoroughly.
Important! To avoid burns, make sure that peroxide splashes do not get into your eyes or mouth.
Hydrogen peroxide + citric acid + soda ash
A solution made from these ingredients will cope even with difficult-to-remove stains, for example, from fuel oil and diesel fuel, and will easily return snow-white laundry.
Pre-remove surface dirt and wash the laundry if necessary. Soak items before washing, especially heavily soiled items, then:
- Heat 2 liters of water.
- Add 1 teaspoon of peroxide, 1 teaspoon of acid and 1 tbsp. spoon of soda.
- Soak soiled clothing for 10–15 minutes.
- Then rinse thoroughly.
Don't keep things in solution for too long
Important! Do not use peroxide with synthetic bleaches. When they are combined, hazardous substances are released.
Detergents for boiling laundry
Many stubborn stains can be removed from fabric using simple substances available in every home. One of them is potassium permanganate. Its dark crystals, which color the water crimson and even violet, are an excellent bleaching agent. Potassium permanganate was widely used in those days when there were no baby diapers. However, its relevance is not lost even today. Linen soiled with the feces of infants or bedridden patients can be cleaned by boiling with potassium permanganate.
To remove stains, crystals are dissolved in water, and the solution is poured into a separate container so that undissolved grains do not spoil the fabric. The pink liquid is poured into a boiling container, 100 g of grated laundry soap or a glass of washing powder is added, water is added to the required level and the laundry is boiled in it. Usually after 1-1.5 hours all stains are washed off.
This method is interesting because it is suitable for different fabrics if boiling is replaced by soaking. Things with stains from grass, rust, ballpoint pen paste, even oil are placed in a solution of soap with potassium permanganate. The soaking time is 8 hours, after which it is enough to rinse the items.
A mixture of baking soda and salt has excellent whitening properties. These completely harmless substances, when boiled, remove old residues from fabric fibers and return them to their lost freshness.
Suitable for boiling and mustard powder. Housewives have long used it to remove greasy stains. For 10 liters of water you will need 4 tablespoons of powder; you need to boil the laundry for 1 hour.
Boiling is an undeservedly forgotten way to restore old things that have lost their appearance. The number of compounds that can be used for it is enormous. The main thing is not to delay. The less worn the laundry, the easier it is to return it to whiteness.
The method that women used to wash sheets and pillowcases, duvet covers and clothes just 30 or 40 years ago is considered by many to be an outdated method, because such work is done by automatic machines. However, when a child appears in the family, young mothers are interested in how to boil laundry. During digestion, harmful microorganisms die, and allergies that are caused by chemical household products do not occur.
Individual cases
Some fabrics require special treatment, while others can easily withstand high temperatures and aggressive detergents.
Linen, cotton
Bleaching cotton and linen items using hydrogen peroxide in an automatic washing machine. To do this, add 10–15 ml of peroxide to the cell with the washing composition. Washing time – 5–7 minutes. The water temperature should be 60–70 °C.
Cotton can also be bleached in the washing machine.
Synthetics
Synthetic fabric interacts well with the acid contained in lemon juice:
- Fill a basin with heated water.
- Squeeze the juice from half a lemon and add 1–1.5 teaspoons of hydrogen solution.
- Soak the clothes for up to half an hour, remove and rinse.
For this bleaching method, the water must be warm.
Make sure that things are completely immersed in liquid when soaking, otherwise there is a high probability of color discrepancy after the procedure.
Delicate fabrics and underwear
Thin underwear and clothes made from delicate types of fabric require special care and treatment. Whiten underwear with peroxide manually.
For this:
- Fill a small bowl with water at room temperature.
- Dissolve 3 tbsp in it. spoons of 3% hydrogen solution.
- Place the laundry in a basin and leave to act for about an hour.
- After the allotted time has passed, take out bras, T-shirts and panties, rinse and send to dry.
Drying white underwear
To bleach delicate fabrics, a solution with the following composition is also used:
- 5 liters of water;
- 1 tbsp. spoon of powder;
- 4 tbsp. spoons of salt;
- 1 tbsp. spoon of peroxide;
- 1 tbsp. spoon of ammonia.
Make sure all components are dissolved, soak the laundry for about 2 hours. Rinse and dry.
Advice! Use only granular powders and liquid gels designed for treating white laundry.
Wool and silk
Items made of wool and silk, as well as items containing this type of fiber, should be bleached by hand washing.
Prepare a solution from the following ingredients:
- 10 liters of water heated to 30–35°C;
- 200–220 g kitchen salt;
- 25–30 g washing powder;
- 600–800 ml of hydrogen peroxide.
If the fabric has an unpleasant odor, wash it with a small amount of powder
Soak silk or woolen items in the prepared liquid for up to 5 hours. After the allotted period of time has passed, take out the clothes, rinse them in a bowl of water at the same temperature, and dry them in a horizontal position.
What happened
It can be assumed that the period of the early Middle Ages is not the object of our attention. Then in Rus', instead of soap, crushed elderberries and soapwort roots mixed with ash lye were used, and the main bleaching agent was the sun - that is, sunlight in the ultraviolet range. By modern standards, these were truly environmentally friendly technologies.
With the discovery of chlorine by Swedish chemist Carl Wilhelm Scheele in 1774, the era of chemical bleaches began. They come in three types: peroxide (peroxide or oxygen-containing), chlorine and sulfur-containing. The use of chemical bleaches is based on the process of oxidation with oxygen of various substances that impart yellowness to the fabric.
The well-known sodium hypochlorite (sodium hypochlorite) has a more than two-hundred-year history of use in fabric bleaching. The historical name of sodium hypochlorite is labarraque water or javel water. Sodium hypochlorite is a chemical chlorine bleach. Chemical formula: NaOCl. Typically, laundries use its aqueous solutions of varying concentrations.
Chlorine bleaches such as sodium hypochlorite, chloramine, bleach, potassium and sodium dichloroisocyanurates work in the cold. Using these cheap bleaches is convenient and simple; they are universal and can be used not only for washing clothes, but for disinfection. However, they also have significant disadvantages: they irritate the skin of the hands and greatly reduce the strength of the fabric. If a drop of chlorine bleach gets on colored laundry, it can result in irremovable stains of the most unexpected color (for example, orange on a dark blue background), and unwashed grease stains in chlorine bleach can turn bright yellow.
Sulfur-containing bleaches (for example, sodium bisulfite-based) release sulfur dioxide when heated with water. Sodium hydrosulfite is used in the textile industry mainly as a reducing agent for vat dyes, for restorative cleaning in polyester dyeing and for removing direct active dyes in cotton dyeing. It discolors not only colored stains of wine, berries and fruits, but also colored products. Sulfur-containing dyes are universal and can be used to process any fabric. However, even the most durable textile dyes cannot resist sulfur dioxide in an aquatic environment. For this reason, sulfur-containing preparations are used extremely rarely in fabric bleaching.
Peroxide bleaches include: hydrogen peroxide (a 30% aqueous solution of which is still sometimes called perhydrol), sodium peroxoborate or peroxocarbonate (persalt), potassium peroxosulfate, carbamide hydroperoxide (hydroperite) - that is, these are compounds in molecules or ions which contain a peroxide fragment of two oxygen atoms connected to each other: -O-O-. When heated in an aqueous environment and decomposed, these substances release atomic oxygen, which oxidizes and discolors contaminants. Peroxide bleaches exhibit their properties most effectively at 80-90°C; even when boiling, they usually do not spoil the colors of the pattern of cotton or linen fabric.
How to restore whiteness to washed items
After a large number of washes, things fade and lose color saturation.
To return washed items to their former whiteness:
- Rub the entire surface of the product with laundry soap.
- Immerse socks and T-shirts in hot water.
- Pour liquid at the rate of 50 ml per 5 liters and stir.
- Leave for 3 hours.
- At the end of the allotted period, remove the laundry.
- Rinse in water, after squeezing the juice from half a ripe lemon into it.
Soap shavings will help restore whiteness to clothes
Rinse first in warm water, then replace the water with cold water.
Advice! Do not bleach frequently, optimally – once every 3-4 washes.
Cleaning gold with stones: advice from a jeweler
There is a fundamental difference in cleaning a smooth ring or a complex piece of jewelry inlaid with stones. To effectively clean gold jewelry with stones at home, it is recommended to use the cleaning methods described above with ammonia alcohol, paste, and liquid soap. As a rule, using a soft brush to clean hard-to-reach areas is indispensable.
Cleaning gold with stones
When cleaning such jewelry, you should take care not only of the condition of the gold, but also of the jewelry inserts. Jewelers recommend using different products for different types of gemstones:
- a ring with topaz or any other gold jewelry with this stone will shine after soaking for 20 minutes in water with a couple of drops of dishwashing detergent diluted in it;
- acids should not be used to clean jewelry with chrysolite;
- earrings with stones such as emerald, ruby or sapphire can be easily cleaned in warm soapy water - hot water is contraindicated for these stones;
- Cleaning gold items with cubic zirconia is done using ammonia, toothpaste without abrasives or any washing powder.
Method for removing yellow stains from white shirts
Very often, owners of white blouses and shirts are faced with the appearance of yellow stains from sweat and marks left by deodorants. Such pollution is striking and looks unaesthetic and repulsive. Fresh stains on white shirts can be removed by washing with regular detergent. But with things that have been lying around for several days, the situation is different.
You need to prepare a bleaching solution:
- heat 4 liters of water to 60–70°C.
- add 2 tbsp. spoons of all-purpose soda ash;
- dilute 2 teaspoons of hydrogen peroxide.
All-purpose soda ash will help whiten a white shirt.
Dip the shirt into the solution for a quarter of an hour, rinse, and dry on hangers.
The juice of 1/2 lemon and a teaspoon of peroxide will help remove yellowness on a synthetic blouse. Use a sponge soaked in this solution to go over the areas of contamination. Or wash the necessary areas with the resulting composition.
If you don’t like the proposed methods, then pay attention to an effective bleach from Aliexpress.
Why do you need to boil laundry?
By boiling you can not only achieve whiteness, this method allows you to get rid of stains. Boiling water helps to disinfect things and cope with many infections. For example, when boiling they die:
- influenza viruses - almost instantly;
- pathogens of hepatitis B and C – in an hour;
- anthrax bacilli and even spores - in an hour;
- spore-forming tetanus bacillus and spores – after 3 hours;
- Staphylococcus aureus, which causes mastitis and severe illness in infants - almost instantly;
- fungi that cause fungal diseases.
Note! Not all pathogens are killed at 100°C, but this method can cope with a wide range of infections. Machine washing at 90°C is not a substitute for boiling, as lower temperatures are lethal to fewer bacteria.
It is useful to treat underwear in this way after a stay in the hospital or maternity hospital. It is recommended to disinfect towels by boiling after visiting a public bath, swimming pool or beach. This method is also good if there is an allergy sufferer in the family who cannot stand the smell of washing powder, because you can boil laundry with soda or laundry soap.
By boiling laundry, you can destroy clothing lice and their larvae - nits. This turns out to be relevant after the child’s stay in the camp where such a problem arose. It will destroy boiling water and dust mites, which thrive in house dust and bedding, causing inflammatory diseases of the skin and eyes.
The disadvantage of boiling is that only products made from natural fibers can be subjected to it. Synthetic fabrics will lose their original structure and become unsuitable for use. Some things made from natural raw materials are also at risk. These include clothing and bedspreads made of wool. An inadvertently boiled sweater can shrink by several sizes or even turn into something like a felt boot.
Important! It is not recommended to test new and good colored items with boiling water. Dyes often do not withstand temperature conditions, the item fades or becomes faded.
However, boiling laundry is useful if a child or adult cannot tolerate washing powders. Allergy sufferers are advised to wear items made from natural fabrics, and many of them can be washed well and bleached by boiling.
Contraindications and overdose
Taking hydrogen peroxide orally is prohibited under the following conditions:
- diabetes;
- ulcer or gastritis;
- pancreatitis;
- during transplantation;
- period of bearing a child;
- lactation period;
- children and teenagers.
Some people, without thinking about the possible consequences, take the solution in unlimited quantities.
In case of overdose, the following conditions and reactions of the body may be observed:
- burn of the mucous membrane of the intestines and stomach;
- blockage of blood vessels;
- vomiting and nausea;
- internal hemorrhage;
- general poisoning;
- allergic reaction in the form of skin rashes or runny nose;
- drowsiness and lethargy;
- burning sensation in the stomach area.
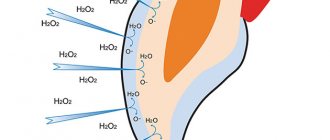
Get rid of bad breath.
Can't get rid of bad breath even after brushing your teeth? An excellent alternative for mouth rinsing is hydrogen peroxide. Since hydrogen peroxide kills bacteria that cause bad breath, all it takes is rinsing your mouth for 30 seconds. You'll be surprised how effective this is. But again, don't overdo it, and use a peroxide rinse once a week, as peroxide kills the good bacteria in your mouth too.
Briefly about the main thing
Products that bleach wood surfaces are divided into chlorine and non-chlorine. The former cope better with fungi, the latter are more often used to restore the color of old wood. But they do not protect it from discoloration in the future. The wood may darken again from ultraviolet rays or wood-staining bacteria. To prevent this from happening, darkened structures need to be treated as early as possible, before the fungus goes deeper, then antiseptically carry out and a protective coating is applied.
Ratings 0
Use in the treatment of stomatitis
If you have ulcers in your mouth, you can speed up healing by rinsing your mouth with hydrogen peroxide. Dilute it with water (1 tablespoon of 3 percent peroxide per glass of water) to avoid causing irritation and blistering (which can happen with high concentrations of hydrogen peroxide). Rinse your mouth with the solution for 30 seconds, spit it out and rinse with plain water.