A problem in which the blood does not clot well is called a bleeding disorder. It is caused by the fact that blood vessels do not normally become blocked when they are damaged.
When everything is fine, when there is bleeding at the wound site, the blood begins to thicken, which prevents large losses. But sometimes this complex mechanism fails, resulting in severe or prolonged bleeding.
When blood does not clot well, this does not always lead to external loss. It can also appear as bleeding under the skin or in the brain.
Causes
Bleeding disorders are divided depending on the etiology - acquired, genetically determined and congenital, as well as autoimmune.
Blood clotting disorders can be caused by hereditary pathologies, but not only. Genetic diseases can also influence the development of the disease. For example, a newborn baby may be diagnosed with hemophilia or von Willebrand disease.
Also, the disorder can be caused by a lack of vitamin K. In addition, such problems can be a consequence of cancer of the liver and other organs.
Most often, bleeding disorders occur due to infectious hepatitis or scarring, which usually occurs with cirrhosis.
Long-term use of strong antibiotics or drugs to treat blood clots can also cause bleeding problems.
4.What can be done at home for hemophilia?
To prevent bleeding and improve your well-being, patients with hemophilia can recommend the following:
- Ask your doctor about how to manage bleeding if you have hemophilia;
- Maintain a healthy weight. Additional stress on joints due to excess weight can cause bleeding in hemophilia;
- Choose forms of physical activity with caution. It is better to give preference to swimming and other sports that do not put unnecessary stress on the joints;
- Consult your doctor before taking any medications. And do not take aspirin, ibuprofen or other non-steroidal anti-inflammatory drugs because they affect blood clotting.
- Organize your living space to avoid injuries and accidents as much as possible.
Symptoms
Main symptoms of the disease:
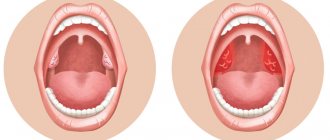
- Skin rashes. Depending on the cause of poor coagulation, both small pinpoint hemorrhages (petechiae) and extensive hematomas may appear on the skin.
- Nosebleeds.
- Hemorrhages in the mucous membranes of the mouth, nose, and intestines. The latter option can lead to the appearance of blood in the stool.
- Hemorrhages in the brain. Occurs with fragility of blood vessels and low blood clotting.
- In the event of an injury, cut, or bruise, the bleeding does not stop for a long time.
- Hemorrhages in joints, muscles and internal organs can be observed with the hereditary disease hemophilia. Bruises (hematomas) can form even with minor mechanical damage.
Impaired hemostasis
General information
The process of hemostasis is able to prevent and stop the flow of blood from the vessel. It is he who ensures the formation of the fibrin clot necessary to restore the integrity of the tissue, and finally removes the fibrin when it is no longer needed. Thanks to this system, blood performs an important function - maintaining a liquid state of blood that flows through the vessels, as well as coagulation when the integrity of the vascular wall is violated. As a result, hemostasis should work to maintain blood volume in the body. This complex system involves platelets, blood cells, extravascular tissues, and the vascular wall. Violation of one of the components leads to disruption of the hemostasis process itself.
Causes of impaired hemostasis:
- DIC syndrome. A common type of hemostasis pathology. Blood clotting is impaired due to the massive release of thromboplastic substances from tissues. May be asymptomatic.
- Impaired hemostasis (coagulopathy). Violation of the functions of the blood coagulation and anticoagulation systems. Pathological condition of the body. It is possible to distinguish immune, acquired and genetic forms of coagulopathies.
- Thrombotic syndrome (thrombophilia). It is characterized by inadequate blood coagulation and thrombus formation, leading to ischemia of tissues and organs.
- Hypocoagulative-hemorrhagic condition. Accompanied by a pathological process of reducing blood clotting.
Classification of hemostasis disorders:
- hereditary or acquired;
- hypocoagulative (decreased blood clotting) or hypercoagulative (increased blood clotting);
- local (thrombosis) or global (DIC syndrome).
Reduced blood clotting
Reduced blood clotting can be manifested by increased bleeding, repeated bleeding, and hemorrhages that occur even with minor injuries. Platelet-vascular hemostasis is disrupted by quantitative and qualitative changes in platelets (thrombocytopenia and thrombocytopathies), as well as lesions of the vascular wall. Thrombocytopenia is a decrease in the platelet count in the blood below normal. However, spontaneous bleeding occurs only when its number decreases to less than 30 G/l. Thrombocytopathies are understood as qualitative inferiority and dysfunction of platelets with normal or reduced levels.
Causes of decreased blood clotting:
- Immune reactions (viruses, drugs);
- Deficiency of cyanocobalamin and folic acid;
- Effect of toxic drugs;
- Hormonal disorders (hypothyroidism);
- Heredity.
One of the possible causes of bleeding may be a decrease in the production of von Willebrand factor by the endothelium of the vascular wall - a large molecular component of blood coagulation factor VIII (hereditary von Willebrand disease). This factor accumulates in platelets and is released during their degranulation.
Hemorrhagic syndrome
Hemorrhagic syndrome is characterized by a state of increased bleeding and disturbances in one of the links of the blood coagulation system. It can be either an acute or chronic disease.
Characteristic symptoms:
- rash in the form of small dots;
- spots up to 3 mm in diameter (petechiae);
- bruises on the skin and mucous membranes (eczymoses);
- spontaneous bleeding.
Ecchymoses are not just bruises, but characteristic signs of hemorrhage into the skin or mucous membrane. If the above symptoms are detected, it is important to conduct laboratory diagnostics. It will show indicators of a decrease in the number of platelets and an increase in the bleeding time.
von Willebrand disease
Von Willebrand disease is a congenital deficiency of von Willebrand factor (VWF) that results in platelet dysfunction. Usually characterized by mild bleeding. There is also an increase in bleeding time, a normal platelet count, and possibly a slight increase in partial thromboplastin time. The diagnosis is based on low levels of von Willebrand factor antigen and abnormal ristocetin cofactor activity. Treatment includes bleeding control with replacement therapy
Symptoms of von Willebrand disease:
- tendency to subcutaneous hemorrhages;
- prolonged bleeding of small cuts;
- prolonged menstruation (in some cases);
- abnormal bleeding after surgery.
Thrombocytopenia due to impaired hemostasis
Thrombocytopenia is characterized by a decrease in the number of platelets and is accompanied by increased bleeding and problems with bleeding. It can accompany any hematological diseases as one of the main symptoms.
As an independent disease, it is sometimes diagnosed as Werlhof's disease . The main causes of thrombocetopenia are impaired platelet production, as well as their increased destruction. Also among the possible causes are infectious and epidemiological :
- HIV;
- hepatitis;
- acute manifestations of herpes infection;
- Infectious mononucleosis;
- ARVI;
- flu.
Impaired hemostasis and the development of bleeding during thrombocytopenia are caused by the following mechanisms:
- increasing the permeability of microvessels for red blood cells and other components of the blood;
- fragility of blood vessels due to wall degeneration;
- decrease in the adhesive-aggregation function of platelets;
- violation of the reaction of release of platelet coagulation factors;
- decreased clot retraction as a result of decreased activity of platelet contractile protein.
Coagulation hemostasis
Along with the primary, vascular-platelet hemostasis, a secondary hemostasis develops - coagulation. It plays the role of additional support and regulation of blood loss from the vessels. Violation of coagulation hemostasis can be caused by the following factors :
- activation of the fibrinolytic system;
- increase in endogenous anticoagulants;
- overdose of anicoagulants.
Anticoagulants inhibit the formation of fibrin threads; they prevent thrombus formation, help stop the growth of existing blood clots, and enhance the effect of endogenous fibrinolytic enzymes on blood clots.
Causes of phase I disorders of blood coagulation
thromboplastin formation may be the following:
- decreased production of factors (IX, X) in liver pathology;
- formation of antibodies in leukemia;
- overdose of the anticoagulant heparin;
- genetic defects in the synthesis of factors VIII, IX and XI.
Causes of phase II coagulation disorders
Causes of violations of thrombin :
- liver diseases;
- hypovitaminoses;
- jaundice;
- enteritis;
- drug dysbacteriosis;
- extensive resection of the small intestine;
- anaphylactic shock;
- heparin overdose.
Causes of phase III coagulation disorders
Hemorrhagic diathesis associated with a violation of the third phase of coagulation - the phase of fibrin , occurs when:
- liver pathologies;
- affected lungs;
- hereditary factor;
- injuries (surgeries) of the lungs, pancreas, burns.
Increased blood clotting
Increased blood coagulation manifests itself at the local level (thrombosis) or manifests itself as generalized intravascular coagulation. The basis of the disease is a violation of platelet-vascular and coagulation hemostasis.
Hypercoagulation can be caused by:
- increased platelet count in the blood;
- weakening of fibrinolysis;
- an increase in platelet content in the blood;
- decreased antithrombotic properties of the vascular wall.
DIC syndrome is a severe disorder of hemostasis that occurs when there is an excessive intake of procoagulants and blood coagulation activators into the blood, which leads to the formation of multiple microthrombi in the vessels of the microvasculature, and then the development of hypocoagulation, thrombocytopenia and hemorrhage as a result of increased functional activity of the anticoagulation system and blood fibrinolysis with subsequent depletion of all systems. The universality and nonspecificity of DIC syndrome is due to the variety of possible factors of its occurrence. Among them, doctors highlight:
- traumatic surgical operations;
- states of shock;
- infections;
- renal failure;
- obstetric ptology;
- intravascular hemolysis;
- terminal states (the process of the body dying).
Treatment of hemostasis disorders
Treatment of the disease is a difficult task for the resuscitator and anesthesiologist. Most patients with hereditary and congenital forms of pathologies undergo treatment and observation by a specialist throughout their lives. Doctors use three basic principles for treating hemostatic disorders.
Etiotropic principle of treatment. It is necessary to exclude or reduce the degree of action of factors causing hemostasis disorders. This may be protection from the following factors:
- physical:
- chemical;
- biological;
- pathological.
Pathogenetic principle of treatment. To prevent the procoagulant activity of platelets, injections of procoagulants and antifibrinolytic drugs are practiced. Transfusions of whole blood, platelets, and protein blood products (fibrinogen, thrombin) are also actively used.
Symptomatic principle of treatment. Solutions are introduced into the patient's body that normalize the rheological properties of blood (blood fluidity). As a result, normalization of the functions of organs and tissues impaired due to microcirculation disorders, bleeding and hemorrhage is achieved.
Treatment
For treatment of this condition to be effective, the causes of the disease must be determined. It is very important to promptly identify and treat the main disorders - liver pathologies or oncological lesions.
Additional therapy methods include:
- injection of vitamin K;
- drugs to improve clotting;
- transfusion of frozen blood plasma;
- Other medications include hydroxyurea and oprelvekin, which help eliminate platelet problems.
The patient's diet should include foods high in calcium, folic acid, vikasol, and amino acids.
These include dairy products: cheese, cottage cheese, kefir. Fish and meat will also help eliminate the symptoms of pathology. It is equally important to eat leafy vegetables - green onions, spinach, cabbage.
Tests and diagnostics
Coagulological screening includes:
- prothrombin index;
- activated partial thromboplastin time;
- amount of fibrinogen;
- platelet count;
- bleeding time.
In case of isolated prolongation of activated partial thromboplastin time, proceed to the second stage of examination:
- do a correction test;
- activity of factors VIII, IX, XI, XII.
If the activity of factor VIII decreases, they proceed to the third stage of examination:
- lupus anticoagulant;
- specific inhibitor of factor VIII.
Regulation of the coagulation system
Figure 6. Contribution of extrinsic and intrinsic tenase to fibrin clot formation in space. We used a mathematical model to investigate how far the influence of a clotting activator (tissue factor) could extend in space. To do this, we calculated the distribution of factor Xa (which determines the distribution of thrombin, which determines the distribution of fibrin). The animation shows the distributions of factor Xa produced by extrinsic tenase (VIIa–TF complex) or intrinsic tenase (IXa–VIIIa complex), as well as the total amount of factor Xa (shaded area). (The inset shows the same thing on a larger concentration scale.) It can be seen that activator-produced factor Xa cannot travel far from the activator due to the high rate of inhibition in the plasma. On the contrary, the IXa–VIIIa complex works far from the activator (since factor IXa is inhibited more slowly and therefore has a greater effective diffusion distance from the activator), and ensures the distribution of factor Xa in space.
[9]
Let's take the next logical step and try to answer the question - how does the system described above work?
Cascade device of the coagulation system
Let's start with the cascade - a chain of enzymes that activate each other. A single enzyme operating at a constant speed produces a linear dependence of product concentration on time. For a cascade of N enzymes, this dependence will have the form tN, where t is time. For the effective operation of the system, it is important that the response is of precisely this “explosive” nature, since this minimizes the period when the fibrin clot is still fragile.
Triggering of coagulation and the role of positive feedbacks
As mentioned in the first part of the article, many clotting reactions are slow. Thus, factors IXa and Xa themselves are very poor enzymes and require cofactors (factors VIIIa and Va, respectively) to function effectively. These cofactors are activated by thrombin, a device where the enzyme activates its own production is called a positive feedback loop.
As we have shown experimentally and theoretically, the positive feedback of factor V activation by thrombin forms the activation threshold - the property of the system not to respond to small activation, but to quickly respond when a large one appears. This ability to switch seems to be very valuable for folding: it helps prevent “false positives” of the system.
The role of the intrinsic pathway in the spatial dynamics of folding
One of the intriguing mysteries that haunted biochemists for many years after the discovery of the essential coagulation proteins was the role of factor XII in hemostasis. Its deficiency was detected in simple clotting tests, increasing the time required for clot formation, but, unlike factor XI deficiency, was not accompanied by coagulation disorders.
One of the most plausible options for unraveling the role of the internal pathway was proposed by us using spatially inhomogeneous experimental systems. Positive feedbacks have been found to be important specifically for the propagation of coagulation. Effective activation of factor X by external tenase on the activator will not help form a clot away from the activator, since factor Xa is rapidly inhibited in the plasma and cannot move far from the activator. But factor IXa, which is inhibited an order of magnitude slower, is quite capable of this (and is helped by factor VIIIa, which is activated by thrombin). And where it is difficult for him to reach, factor XI, also activated by thrombin, begins to work. Thus, the presence of positive feedback loops helps create the three-dimensional structure of the clot.
Protein C pathway as a possible localization mechanism for thrombus formation
Activation of protein C by thrombin itself is slow, but accelerates sharply when thrombin binds to the transmembrane protein thrombomodulin, synthesized by endothelial cells. Activated protein C is capable of destroying factors Va and VIIIa, slowing down the coagulation system by orders of magnitude. The key to understanding the role of this reaction was spatially inhomogeneous experimental approaches. Our experiments suggested that it stops the spatial growth of the thrombus, limiting its size.
Summarizing
In recent years, the complexity of the coagulation system has gradually become less mysterious. The discovery of all essential components of the system, the development of mathematical models and the use of new experimental approaches made it possible to lift the veil of secrecy. The structure of the coagulation cascade is being deciphered, and now, as we saw above, for almost every significant part of the system, the role it plays in the regulation of the entire process has been identified or proposed.
Figure 7 shows the most recent attempt to reconsider the structure of the coagulation system. This is the same diagram as in Fig. 1, where parts of the system responsible for different tasks are highlighted with multi-colored shading, as discussed above. Not everything in this scheme is securely established. For example, our theoretical prediction that activation of factor VII by factor Xa allows clotting to respond in a threshold manner to flow rate remains as yet untested experimentally.
Figure 7. Modular structure of the coagulation system: the role of individual coagulation reactions in the functioning of the system.
[1]
It is quite possible that this picture is not yet completely complete. However, progress in this field in recent years gives hope that in the foreseeable future, the remaining unsolved regions of the coagulation circuitry will gain meaningful physiological function. And then it will be possible to talk about the birth of a new concept of blood coagulation, which replaced the old cascade model, which faithfully served medicine for many decades.
The article was written with the participation of A.N. Balandina and F.I. Ataullakhanova and was originally published in Priroda [10].
List of sources
- Fatkullin I.F., Zubairov D.M. Hereditary and acquired defects of the hemostatic system in obstetric and gynecological practice. M., 2002. P. 64.
- Galstyan G.M., Sukhanova G.A. Introduction to hemostasis, modern blood products and their effect on coagulation // Medical Council. 2013. from 11-13.
- Barkagan Z.S. Diagnosis and controlled therapy of hemostasis disorders / Z.S. Barkagan, A.P. Momot. – M.: Newdiamed, 2001. – 296 p.
- Barinov S.V., Dolgikh V.T., Medyannikova I.V. Hemocoagulation disorders in pregnant women with gestosis. Journal of Obstetrics and Women's Diseases. – 2013; 62 (6): 5–12.
- Degtyarev D.N., Karpova A.L., Mebelova I.I. and others. Draft clinical recommendations for the diagnosis and treatment of hemorrhagic disease of newborns // Neonatology. 2015. No. 2. P. 75–86.