A beautiful smile is a natural desire of people, regardless of age and gender. In pursuit of aesthetics, many practice using advertised fluoridated toothpaste to whiten and strengthen teeth. But mass market products do not always live up to expectations. If you want a healthy smile, professional help in solving problems related to dental health, you will receive at the Nurimed dental clinic in St. Petersburg. You can make an appointment with a doctor by calling.
The role of fluoride ions in the human body
Fluorine plays an important role in the functioning of the human body. The microelement is necessary for the formation of the skeleton, maintaining the strength of bones and teeth. It determines the condition of nails, hair, and is involved in hematopoiesis.
Let us designate other functions of the microelement:
- promotes restoration of bone tissue after damage;
- stimulates the immune system;
- removes heavy metals and radioisotopes;
- ensures the absorption of iron.
In the human body, fluorine is concentrated mainly in bone and dental tissue, dentin.
Harm of fluoride
Its negative impact on the body in case of excess is reflected by the development of many reactions. Thus, in children, this condition can cause speech impairments, significantly reduce mental abilities to study mathematics and foreign languages, and impair memory.
IT IS IMPORTANT TO KNOW! In adults, high fluoride levels can cause hormonal changes. Its ions, accumulating in the thyroid tissues, reduce testosterone levels, which, in turn, can cause many abnormalities, including infertility.
Fluoride poisoning: symptoms and prevention
Sodium fluoride, along with substances such as arsenic, mercury and lead, is included in the category of heavy metals. These dangerous compounds enter the human body through food, water, inhalation of contaminated air or vapors emanating from various chemicals and cleaning products.
The human body absorbs about 2 kg of toxins annually. Some of them are successfully neutralized and eliminated by the body, but some have the ability to accumulate in internal organs. This can lead to the development of a number of diseases, constant pain and allergies.
Poisoning caused by fluoride compounds is not one of the diseases often encountered in medical practice. However, given the consequences, they should not be written off.
In this case, a malfunction of the central nervous system occurs. It manifests itself as: causeless anxiety, convulsions, paralysis of muscles and the vasomotor center, respiratory disorders (shortness of breath), increased intestinal motility. What happens from an overdose of glycine?
Symptoms from the gastrointestinal tract: excruciating, spasmodic pain, profuse salivation, attacks of nausea, slight vomiting and loose stools.
When air with high concentrations of fluoride compounds is inhaled, toxic pulmonary edema can occur. But not only the upper respiratory tract is damaged, but also the eyes. They quickly turn red and profuse lacrimation begins. A persistent cough, frequent bronchitis and pneumonia may also be signals of regular and unnoticeable fluoride poisoning.
Treatment of poisoning with heavy metals, including fluoride compounds, should be carried out exclusively in a specialized medical facility.
Such conditions manifest themselves with the regular intake of fluorine and its compounds into the human body. And also during a single unprotected contact with substances that contain this element.
To prevent the accumulation of sodium fluoride and heavy metals in the body, you must:
- Maintain adequate iodine levels.
It plays an important role in cell metabolism and is an element necessary for the normal functioning of the thyroid gland, which, among other things, produces a hormone that promotes the proper formation and renewal of bone tissue. - Consume foods high in boron.
It helps in removing excess fluoride from the body. This element is found in nuts, plums, broccoli, bananas, avocados and honey. - Calcium and magnesium inhibit the absorption of fluoride by cells. They can be taken in the form of pharmaceutical preparations or in natural form. They are found in nuts, dark green vegetables, fish, beans, avocados, dairy products, dried fruits and dark chocolate.
- Once a year, check tap water for fluoride content.
- Visit your dentist regularly. This is especially true for preschool children. At the slightest sign of fluorosis, change oral hygiene products to ones that do not contain fluoride.
If you take into account all the nuances, hygiene products with sodium fluoride can be excellent helpers in the fight for the beauty and health of your teeth. If you are afraid of the consequences, then choose alternative products.
How to remove excess substance from the body
There are methods that allow you to remove excess fluoride from the body, but they give the desired result only if the pathology is in the initial stages of its development. Here are the main ways to combat intoxication with this element:
- iodine – promotes the excretion of the substance in the urine, so it is recommended to increase the consumption of foods rich in this component: strawberries, lingonberries, beans, potatoes,
- boron – also effectively removes fluoride and is found in high concentrations in honey, nuts, dates, avocados, prunes,
- selenium – blocks the activity of an excess element, found in sufficient quantities in Brazil nuts,
- dry sauna – helps remove accumulated toxic substances through sweating.
Consumption of iodine-containing products helps remove the substance from the body.
But before moving on to measures to remove the substance, it is necessary to limit its entry into the body. For a while, you will have to give up foods with a high content of it, switch to clean bottled water, and stop using fluoride-containing hygiene products.
Benefits of fluoride for teeth
The positive impact of microelements on the condition of teeth is difficult to overestimate. This is why fluoride toothpaste is widely advertised and recommended by dentists. The benefits of microelements for dental health are as follows:
- Fluorides improve metabolism.
- Fluoride stimulates the production of the salivary gland and slows down the formation of tartar.
- Fluoride ions inhibit the activity of harmful microorganisms in the oral cavity and prevent their destructive effects on tooth enamel.
- The trace element promotes tissue remineralization, i.e. allows you to restore damaged areas of teeth.
- Fluorides provide caries prevention.
The effect of fluoride on dental and oral health
Data from numerous scientific studies leave no doubt about whether fluoride is beneficial for teeth. First of all, it protects them from caries and maintains oral health. The presence of ions of this element in saliva is critically important. The fact is that they have the ability to be embedded in the crystal lattice of hydroxyapatite, a compound that forms the basis of tooth enamel [12].
Due to this and other mechanisms of action of fluoride ion:
- prevents demineralization (loss of minerals) of enamel and strengthens it, protecting teeth from acid damage and caries [12];
- promotes remineralization of enamel, that is, helps restore the content of minerals in it and eliminate defects on its surface [12];
- suppresses the growth of pathogenic bacteria in the oral cavity [13];
- reduces acid production by pathogenic bacteria in dental plaque [14].
It was also found that in addition to systemic (internal, coming from water or food), oral health is also effectively protected by local fluoridation [12]. This is the name of a method in which fluoride is applied directly to the tooth surface, without swallowing the drug. For this purpose, fluoride-containing toothpastes , mouth rinses, and professional dental fluoride varnishes are used [12].
Is fluoride in toothpaste harmful?
When it comes to the benefits or harms of a microelement for the body, it is important to understand that the same substance can have positive and negative effects. It is not the substance itself that causes harm, but its excessive concentration in the body.
The harm of an excess of fluoride is manifested by metabolic disorders, deterioration of blood clotting, fluorosis, or as it is also called, speckled enamel syndrome. This is a dental disease that causes light stains to form on the teeth. Over time, they become darker, turn yellow and lead to the destruction of tooth enamel. The disease requires treatment. Typically, remineralization therapy, photophoresis, and professional bleaching are used for this.
Fluorine is the 9th element of the periodic table
Fluorine is a chemical element from the halogen group. Actively contacts with other substances. As a result, bright reactions appear: combustion, explosion, boiling. Research by dentists has revealed the presence of fluoride at approximately 0.02% in tooth enamel. The population of regions where the water contains insufficient amounts of fluoride is more susceptible to caries. At the same time, its excess is manifested by darkening of the teeth and the presence of white spots on them (fluorosis). Over time, the enamel begins to gradually deteriorate.
How much fluoride does a person need and what foods contain it?
The daily dose of fluoride required for an adult is 0.03 mg/kg. During the period of formation and growth of the skeleton, the child’s body needs a larger amount - 0.15-0.1 mg/kg.
You can get this microelement from foods that are present in your daily diet:
- fish (the best source is mackerel, pollock, cod);
- all types of meat and offal;
- dairy products;
- raw nuts: walnuts, cashews, hazelnuts;
- fruits and vegetables (pumpkin, grapefruit, apple);
- mushrooms (ceps, chanterelles).
A balanced diet allows you to get fluoride from foods that are always in the refrigerator.
Other substance resources
- In cities, fluoride has already been added to tap water. Although the dosage is quite small. It is also found in the air and soil in some regions. The local population here should use fluoride-free toothpaste. Otherwise, there is a chance of getting poisoned, the consequences of which are very serious. It is known that the 9th element is capable of accumulating in the body, gradually poisoning it.
- A safe dose of halogen for an adult is considered to be from 0.5 to 5 mg per day. It is necessary to take into account the total number of sources of the substance. At the same time, ingesting fluoride compounds is dangerous, and external use of a minimal amount of fluoride in toothpaste is absolutely safe.
- Many food products contain fluoride. It is found in varying quantities in sea and river fish, meat and dairy products, apples, nuts and onions. Rice, buckwheat, oatmeal, and wholemeal flour are fluoride-containing products. Green and black tea contains fluoride.
- Prescribed therapy for diseases of bones and joints in adults and children significantly enriches the body with fluoride.
The conclusion is this: if a person eats well and consumes a sufficient amount of fluid, additional supply of fluoride to the body is not required. In this case, teeth and bones will be strong and healthy.
Fluoride-containing hygiene products:
- COLGATE maximum protection against caries;
- PARADONTAX;
- BLEND-A-MED active fluorine;
- New fluorine pearls.
Water fluoridation
Saturation of water with fluoride ions is the practice of most countries in Europe and the post-Soviet space. By drinking about two liters daily, you can fully provide your body with this microelement.
But there are also opponents of fluoridation. First of all, a negative attitude towards the saturation of water with microelement ions is associated with the accumulation of fluoride in the body. They are difficult to remove and can have negative effects in the future. It is important to understand the saturation level of the water. Fluoridated water poses a real health hazard only when toxic fluorine compounds are used or the microelement concentration exceeds 1 mg/l of water.
Big fluoride conspiracy - why toothpaste is dangerous
How often do you brush your teeth? Most likely once or twice a day. How often have you thought about what substances are in the toothpaste that you use day after day, month after month? Probably the same as me - never. Just like me, you rely on the recommendations of the Dental Association. And in vain.
After I came across information about the dangers of fluoride, I really thought about the fact that I had never even thought about reading the composition of toothpaste on the box before. Not to mention learning more about the components themselves. Frankly, I learned all the information about fluoride from advertising posters and videos and sometimes from dentists.
To my surprise, when I searched, I found so many “interesting” things about fluoride and the effect of this substance on the body that I am still in slight shock. This article is food for thought.
What is fluorine and fluoride
Fluoride is a fluoride ion. All organic and inorganic compounds containing fluorine are fluorides, which will be discussed in this article. Fluorine is a gas, and in nature it is most often found in compounds with other substances, for example calcium fluoride (CaF) or sodium fluoride (NaF).
Fluoride is a natural element that is part of the earth's crust. It is therefore natural that a small dose of fluoride (considerably less than 1 ppm) is found in natural water. Plants naturally absorb fluoride from soil and water, so small amounts of fluoride are present in all our food and water, and also accumulate in animal tissue and plants.
Although fluoride is a natural substance, it is toxic to humans, much more toxic than lead. An injection of 2-5 grams of sodium fluoride (a standard ingredient in toothpaste) is a lethal dose. The amount of fluoride in one medium-sized tube of toothpaste is enough to kill a small child if the entire tube is used at once. Fluoride toothpaste contains a much higher concentration of fluoride compared to naturally occurring fluoride.
Fluoride was originally added to water because it was believed that fluoride was extremely beneficial for dental health and prevented tooth decay. And only then into toothpaste. In some countries, such as the USA, about 2/3 of all natural water is fluoridated.
How does fluoride work in the fight against tooth decay?
Fluoride is believed to be toxic to bacteria. Bacteria, like all living forms, also feed, and use sugar as food (glucose, sucrose, fructose, lactose or food starches) and bacterial waste that can dissolve tooth enamel and are the very acids that cause tooth demineralization or caries . Fluoride poisons the bacteria, reducing its ability to process sugar. Unfortunately, fluoride is so poisonous that when used, it poisons not only bacteria, but also other cells.
Risks of Fluoride Use
Fluoride can cause serious health problems even when consumed in small doses, such as those found in toothpaste or fluoridated water.
Fluorosis is chronic fluoride intoxication. There are two types: dental and skeletal, it is expressed in terrible symptoms, I won’t even describe them.
There are also over 30 animal studies that suggest fluoride is a neurotoxin that reduces cognitive abilities (language learning, speech, thinking ability) and memory. Essentially, fluoride makes you dumber.
Now strawberry, albeit bitter.
Why did people start adding fluoride to toothpaste and water?
As always, big money and politics are involved in this story. The history of the creation of the myth about the benefits of fluoride is described in the book “Fluoride Deception”, published by the famous journalist and BBC producer Christopher Bryson, which is based on a 10-year study of facts and rumors on the topic of fluoride. In this book, Bryson profiles some of the most important individuals and institutions that have played a major role in bringing fluoride to the use of dental disease in the United States and around the world.
Defenders of the fluoridation theory say that in relation to fluorine there are two different issues that do not overlap. The first is due to the fact that fluorides are an industrial waste from metal production, and the second question concerns the usefulness of fluoride in dental hygiene products. This is not true, since both of these two storylines are tightly intertwined from the very beginning.
So, about the beginning of the story. The first statement that fluoride was beneficial to dental health and that it should be added to drinking water to prevent dental disease was made by one researcher, Dr. Gerald Cox of the Melon Institute in Pittsburgh. Cox began research on fluoride at the suggestion of Francis Frery, director of the research laboratory of the American Aluminum Company, who was apparently very concerned about the large problem of air and environmental pollution in the vicinity of aluminum smelters and the negative effects of fluoride on the health of plant workers.
It must be understood that the Melon Institute served as the main advocate for all large companies in the metal processing industry, so it is absolutely no coincidence that such a proposal came from a researcher at this institute.
At that time, in the period from 1956-1968, more lawsuits were filed in court for harm caused to health by fluorine alone than for the other 20 (!) pollutants combined. There was definitely a pressing need for some way to defend against so many lawsuits, and it would have been a good idea to have a theory based on actual research that fluoride was good for your health.
Another advocate of fluoridation was Harold Hodge, one of the most influential and senior physicians and researchers. This man enjoyed unquestioned authority among those in power in the field of health care and published more than one work in support of the water fluoridation program, the introduction of which was considered in 1957.
It is now known that Hodge was one of the organizers of an experiment to study the effects of radiation on the health of people who were inoculated with plutonium.
What connection?
Straight. He served as chief toxicologist for the Manhattan Project. The goal of this project was to develop an atomic bomb, which was later dropped on Jeroshima and Nagasaki. Hodge investigated the toxicity of all the chemicals that were used in the production of the atomic bomb and fluorides were the main problem because. They were used in incredible quantities when creating the bomb.
The documents Bryson discovered clearly stated that Hodge was being tasked with providing information that could help the government and military defend against personal injury lawsuits. And on the contrary, all information that can be used against the army must be deleted.
If it were recognized that water fluoridation was harmful, all organizations working with fluoride, including the Nuclear Energy Commission, the government and the US military, would be subject to countless lawsuits. In other words, there was not a single chance that Harold Hodge would have framed such powerful organizations.
At the same time as Hodge, a famous physician and promoter of the fluoridation theory, Dr. Keyhoe published a large scientific work on the beneficial effects of fluorides. This work was sponsored by the following organizations:
Aluminum Company of America (ALCOA), Aluminum Company of Canada, Fuel Research Institute of America, DuPont, Kaiser Aluminum, Reynolds Metals, United Steel, National Institute of Dental Research (NIDR). Keyhoe's personal files contain references to collaborations with the Fluoride Legality Committee, to which Keyhoe provided materials to defend corporate clients (listed above) against fluoride-related lawsuits.
On top of that, what helped sell fluoride to the entire nation was none other than PR's father, Edward Bernays, the nephew of Sigmund Freud, who was a true evil genius and a professional at creating an attractive image for harmful products. Oscar Ewing's brother, Edward L. Bernays, was a good psychologist and the nephew of Sigmund Freud. Edward conducted research on controlling the human mind, or rather society. He even published a book called “Propaganda.” In addition to popularizing fluoridation, Bernays participated in the propaganda of cigarettes. Bernays was invited by NIIOS to help carry out PR for the nation's fluoride. Their plan was to convince dentists that fluoride was good for teeth, and then the dentists would “sell” fluoride to everyone else.
For decades, the benefits of fluoride have been promoted to the public, starting in school. Scientists who stated that instead of being beneficial, fluoride had a strong negative effect on the human body were fired, persecuted, and ridiculed in the press. Only recently have some scientists been able to publish the results of studies showing the dangers of sodium fluoride when used even in doses allowed by standards.
It is not difficult to guess that the toothpastes that have been most widely advertised (Colgate, Blend-a-med, Aquafresh, etc.) have the highest fluoride content. People began to buy these toothpastes not because their benefits were proven, but because the repeated lies (in the form of advertising) began to be perceived by many people as the truth. This psychological technique was used to widely promote fluorine to the masses.
What to do now?
To begin with, you must look at this issue with “open eyes” (it would be a good idea to involve your brain) and make your own conscious decision. Common sense dictates that you should not take (especially regularly) any substance unless you fully understand what it is.
My opinion is that if there is even a slight suspicion that fluorides may be harmful, there is no point in using them. In this case, a huge amount of materials convinces us that it is better to abandon it.
In addition, here is what dentists advise for “fluoride-free” caries prevention:
The less white artificial sugar is in the food you eat, or the less often you eat foods rich in white sugar, or the less time you allow table sugar to remain in your mouth, the less acid the bacteria will produce.
It is better to consume fructose rather than white sugar. Or even better - consume sugar only in whole foods - fruits, dried fruits, nuts. And as a sweet seasoning you can use cinnamon, turmeric, etc. Beware of using the genetically modified sweetener aspartame. It is even more harmful than white sugar.
It is recommended to reduce the amount of time sugar spends in your mouth. After eating sugar-rich foods, brush and floss your teeth, or at least rinse your mouth.
It is very harmful to suck sweets in your mouth and drink sugary drinks for a long time. If you still have to drink sweet water (for example, a honey drink), then after it you should quickly brush your teeth.
It is recommended that you floss and brush your teeth frequently and thoroughly.
It is recommended to floss and brush your teeth after every meal—even small amounts. It is recommended to spend a little more time caring for your teeth - it is important to clean them as thoroughly as possible. Areas that you can't reach with a brush or floss are most likely to form cavities.
Additional Information:
Countries that have stopped, rejected or banned water fluoridation: Austria, Belgium, China, Czech Republic, Denmark, Finland, France, Germany, Hungary, India, Israel, Japan, Luxembourg, Holland, North. Ireland, Norway, Scotland, Sweden, Switzerland.
According to Dr. R. Carton, a former EPA scientist, "Fluoridation is the greatest scientific fraud of our century, if not of history." Most studies point to serious health risks from fluoride: it can cause illness, birth defects and premature death.
Dean Burke, a former chemist at the US National Cancer Institute, states that "fluoridation causes more cancer deaths than other chemicals."
Dr. A.E. Bannik, in his book “Choice Purity,” states: “Fluoridation of drinking water is criminal, extremely unscientific, and it is chemical warfare. Not only does fluoride not strengthen your teeth, it also hardens your arteries and brain.” Fluorine is obtained by washing the airborne fluoride emissions from molten metals or plants fertilized with phosphates.
Today, people ingest vast amounts of fluoride from a variety of sources. Fluoride is not only in toothpastes, but also in water, drinks, juices, and in all products prepared with fluoridated water. Dental fluorosis, fluoride poisoning, is characterized by the speckling and softening of tooth enamel. 60% of children have these symptoms. Don't use fluoride toothpastes. Much better than paste with propolis, myrrh, baking soda or tea tree oil
Dr. J. Yamouyiannis, in his book Fluoride Factor in Aging, writes: “With an ally like truth, it is easy to win. The truth is that fluoridation is chronically poisoning millions.” This distinguished biochemist was the biochemistry editor of the Chemical Abstracts Service, the world's greatest chemical information center. When he began to question the safety of fluoridation, he was told to shut up: millions of dollars in federal investment were at risk. Within weeks he was forced to resign.
A good toothpaste features ultra-fine silica powder, which is great for removing stains from teeth and also polishes teeth without damaging the enamel. The choice is yours.
Fluorophosphate, sodium fluoride, sodium monofluorophosphate are absent in such toothpastes as: “New Pearl with Calcium” (Russia), “Forest Balsam”, “Cedar Balsam”, “Splat”, “Sunshine Brite”, “ROCS” (Switzerland- Russia).
Fluoride compounds in modern toothpastes
Fluoride is an important element in toothpaste, but not every fluoridated toothpaste is good for your teeth. When choosing a product, pay attention to the composition:
- Tin fluoride. The compound has a high remineralizing effect on the enamel, but it also has side effects: darkening of the enamel, discoloration of fillings, deterioration of the gums. Manufacturers of quality toothpastes do not use this compound (among well-known brands, Colgate continues to use the compound today in some types of toothpastes).
- Sodium monofluorophosphate. The disadvantage of this compound is the slow release of active fluorine. The process begins only after three minutes of brushing your teeth. Prolonged mechanical action is not beneficial for the enamel, so a paste with this compound, if the rules of oral care are followed, is not effective.
- Sodium fluoride. Active fluorine is released quickly, so pastes containing it have a good remineralizing effect.
- Aminofluoride. The most effective compound that helps restore enamel and form a protective film on its surface. Even after brushing your teeth, microelement ions continue to enter the enamel, thereby strengthening it.
When choosing a paste, give preference to products containing sodium fluoride or amino fluoride.
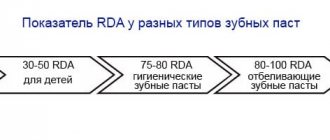
The concentration of the substance plays an equally important role. On toothpaste tubes it is indicated as a percentage. Sometimes, instead of percentage content, you can see the “ppm” index, which reflects the degree of concentration of the microelement in the product. 0.1% corresponds to an index of 1000 ppm.
The higher the concentration, the more pronounced the restorative effect of the paste. Prophylactic pastes contain from 950 to 1150 ppm. Effective fight against caries is possible with a fluoride content of 1000 ppm. Treatment pastes contain at least 1350 ppm.
Preventive pastes can be used by everyone, even children and pregnant women. At the same time, the European Academy of Pediatric Dentistry has established acceptable standards for microelement content in the product: for children under 2 years of age - no more than 500 ppm, for children under 6 years of age - 1000 ppm. The duration of use of toothpaste is determined by the dentist, taking into account the condition of the teeth, diet, quality and quantity of drinking water consumed.
Fluoride analyzers
Halogen analyzers are used to determine the fluoride content in toothpaste. The concentrations of any halogens and sulfur in samples can be accurately measured using a combustion product ion chromatography analytical system consisting of an ion chromatograph, automatic oven model AQF-100/AQF-2100H. The device was developed by Mitsubishi Chemical Analytech. The installation has an automatic sample feeder and a pyrolytic tube heated by an oven. It allows you to determine fluoride concentrations in toothpastes, effectively decomposing samples into volatile components. The resulting gases containing fluorides are absorbed in a special trap with an absorption solution and then analyzed on an ion chromatograph. This method allows you to obtain the most reliable results of fluorine analysis and find out how much it is contained in the test sample.
Choosing a brush - expert advice
Another mandatory attribute of daily oral care is a brush. Her choice also needs to be taken seriously, and dentists give the following advice on this matter:
- the head should not exceed 3 cm in length,
- it is better to choose models with artificial bristles, since natural ones create favorable conditions for the growth of bacteria,
- The ribbed back surface of the head is designed to clean the tongue - there is no need to additionally purchase a special scraper.
It is important to choose the right toothbrush.
If you have sensitive gums that are prone to inflammation and bleeding, it is better to give preference to a brush with soft bristles. But keep in mind that it will be worse at cleaning the enamel from plaque, so the procedure must be especially intense. Hard brushes are intended to be used according to indications - otherwise they can injure the gums and enamel and do more harm than good.
Sources:
- https://myecotest.com/vred-ftora-v-zubnoy-paste/
- https://Ru-organic.com/info/zubnaya-pasta-s-ftorom.html
- https://asepta.ru/spravochnik/zubi/zubnye-pasty-s-ftorom/
- https://DentConsult.ru/sredstva/ftorsoderzhashie-zubnye-pasty.html
- https://himya.ru/ftoridy.html
- https://FB.ru/article/254057/ftor-v-zubnoy-paste-polza-i-vred-chem-i-kak-pravilno-chistit-zubyi
- https://poleznii-site.ru/dom/byt/vreden-li-ftor-v-zubnoy-paste-ego-rol-v-organizme.html
- https://24stoma.ru/lechebnaya-zubnaya-pasta-ot-kariesa.html