Naturally occurring mineral form of calcium apatite
| Hydroxyapatite | |
| Hydroxyapatite crystals on a matrix | |
| General | |
| Category | Phosphate mineral apatite group |
| Formula (repeating unit) | Ca5(PO4)3(OH) |
| Strunz classification | 8.BN.05 |
| Crystal system | Hexagonal |
| Crystal class | Dipyramidal (6/m) HM Symbol (6/m) |
| Space group | P 6 3/m |
| Cell | a = 9.41 Å, c = 6.88 Å; Z=2 |
| Identification | |
| Mass formula | 502.31 g/mol |
| Color | Colorless, white, gray, yellow, yellowish green |
| Crystal Habit | In the form of lamellar crystals and stalagmites, concretions, from crystalline to massive crusts. |
| Split | Bad on {0001} and {10 1 0} |
| Fracture | Conchoidal |
| Perseverance | Fragile |
| Mohs hardness | 5 |
| Shine | Glassy to semi-resinous, earthy |
| Band | white |
| Transparency | Transparent to translucent |
| Specific gravity | 3.14–3.21 (measured), 3.16 (calculated) |
| Optical properties | Single axis (-) |
| Refractive index | n ω = 1.651 n ε = 1.644 |
| Birefringence | δ = 0.007 |
| Recommendations | [1] [2] [3] |
Hydroxyapatite Needle-shaped crystals of hydroxyapatite on stainless steel.
Scanning electron microscope image from the University of Tartu. Nanoscale Ca-HAp coating, image obtained with a scanning probe microscope. 3D rendering of half a hydroxyapatite unit cell from X-ray crystallography. Hydroxyapatite
, also called
hydroxyapatite
(
HA
), is a natural mineral form of calcium apatite with the formula Ca 5 (PO 4 ) 3 (OH), but is usually written Ca 10 (PO 4 ) 6 (OH) 2 to indicate that the unit cell of the crystal consists of in two parts. [4] Hydroxyapatite is the terminal hydroxyl element of the apatite complex group. The OH ion can be replaced by fluoride, chloride, or carbonate to form fluorapatite or chlorapatite. It crystallizes in the hexagonal crystal system. Pure hydroxyapatite powder is white in color. However, naturally occurring apatites can also be brown, yellow, or green in color, comparable to the discoloration seen in dental fluorosis.
Up to 50% by volume and 70% by mass of human bone is a modified form of hydroxyapatite, known as bone mineral. [5] Calcium-deficient carbonated hydroxyapatite is the primary mineral that makes up tooth enamel and dentin. Hydroxyapatite crystals also occur in small calcifications in the pineal gland and other structures known as corpora or "brain sand." [6]
Calcium-deficient hydroxyapatite[edit]
Calcium-deficient (non-stoichiometric) hydroxyapatite, Ca 10− x
(PO 4 ) 6−
x
(HPO 4 )
x
(OH) 2−
x
(where
x
is between 0 and 1), has a Ca/P ratio of 1.67 to 1.5.
The Ca/P ratio is often used when discussing calcium phosphate phases. [9] Stoichiometric apatite Ca10(PO4)6(OH)2 has a Ca/P ratio of 10:6, usually expressed as 1.67. Non-stoichiometric phases have a hydroxyapatite structure with cation vacancies (Ca 2+ ) and anion (OH - ) vacancies. The sites occupied exclusively by phosphate anions in stoichiometric hydroxyapatite are occupied by phosphate or hydrophosphate anions HPO 4 2-. [9] The preparation of these calcium-deficient phases can be obtained by precipitation from a mixture of calcium nitrate and diammonium phosphate with the desired Ca/P ratio, for example to obtain a sample with a Ca/P ratio of 1.6:[10] 9.6 Ca(NO 3 ) 2 + 6 (NH 4 ) 2 HPO 4 → Ca 9.6 (PO 4 ) 5.6 (HPO 4 ) 0.4 (OH) 1.6
Sintering of these non-stoichiometric phases leads to the formation of a solid phase, which is a homogeneous mixture of tricalcium phosphate and hydroxyapatite, called biphasic calcium phosphate: [11]
Ca 10−
x
(PO 4 ) 6−
x
(HPO 4 )
x
(OH) 2−
x
→ (1 -
x
) Ca 10 (PO 4 ) 6 (OH) 2 + 3
x
Ca 3 (PO 4 ) 2
Procedure for injecting filler
In modern cosmetology, the use of fillers is one of the safest, most effective and fastest methods for solving aesthetic and medical problems. Contour plastic surgery using these drugs can only be performed by a specialist who has been trained in a special program and has a certificate giving him the right to use this product in practice. These are the ones who work at the VIRTUSCLINIC Center for Aesthetic Cosmetology.
On average, the procedure lasts 30 minutes and is performed under local anesthesia. The doctor cleans the facial surface and determines the areas where the drug will be administered, leaving markings there in the form of dots. Some specialists take photos before and after the procedure.
The cosmetologist demonstrates the drug to the patient in its entire packaging, with the obligatory indication of its expiration date and dosage. The filler is injected using a very thin needle subcutaneously in the previously designated area. The specialist chooses the method and methods of administering the drug independently, taking into account all the features of the procedure and the patient.
The session ends with a light massage, which helps to evenly distribute the filler under the skin.
The effect of the procedure is noticeable immediately after it is performed, but the maximum result usually appears after 10–14 days.
Biological function[edit]
Mantis shrimp[edit]
Clubbed
the appendages
of Odontodactylus scyllarus
(peacock mantis shrimp) are made of an extremely dense form of the mineral that has a higher specific strength; this led to its investigation for possible synthesis and engineering uses. [12] Their dactylic spines have excellent impact resistance due to the fact that the impact area is composed primarily of crystalline hydroxyapatite, which has significant hardness. A periodic layer beneath the impact layer, consisting of hydroxyapatite with lower calcium and phosphorus content (resulting in a much lower elastic modulus), inhibits crack growth by causing new cracks to change direction. This periodic layer also reduces the energy transferred through both layers due to the large difference in moduli, even reflecting some of the incident energy. [13]
Mammal/primate/human[edit]
Hydroxyapatite is present in bones and teeth; Bone is composed primarily of HA crystals embedded in a collagen matrix—65 to 70% of bone mass is HA. Similarly, HA constitutes 70 to 80% of the mass of dentin and enamel of teeth. In enamel, the matrix for HA is formed by amelogenins and emelins instead of collagen. [14]
Hydroxyapatite deposits in tendons around joints cause calcific tendinitis. [15]
Indications for use
Cosmetologists sometimes call preparations containing calcium hydroxyapatite nasolabial fillers. This is due to the fact that they give the most positive results in this zone. However, with the help of such fillers, other parts of the human body are also corrected:
- Temple area. Aging is an inevitable process for a person, which is accompanied by the loss of the soft tissues of the face of their previous volume. This leads to the appearance of so-called shadows or depressions in the temple area. Lifting filler, which is based on calcium hydroxyapatite, replenishes and corrects the volume lost in these problem areas, which confirms the high effectiveness of the drug;
- cheekbones. They play one of the main roles in the aesthetic formation of the face and are responsible for the perception of it by the people around them. A person with clearly defined cheekbones looks young and fresh, and facial features acquire special sophistication. With the help of preparations with calcium hydroxyapatite, it is possible to carry out a thorough correction of this area of the face, restore the volume and relief of the cheekbones;
- nose area. Many people are unhappy with their nose, and this does not depend on age at all. Cosmetologists recommend that such patients undergo rhinoplasty without surgery. In this case, the filler acts as a kind of implant. With its help, you can make your nose straighter, hide a hump, adjust the tip, etc. Correction of the nose with fillers makes sense for the correction of minor defects. It is impossible to radically change the shape of this organ or reduce its size using rhinoplasty;
- nasolabial folds. This area is considered one of the most problematic and noticeable. With age, the folds that stretch from the edges of the nose to the corners of the lips become deep and distinct, which gives the face a gloomy, sad appearance. Tightening them with creams or using facial skin tightening is almost impossible. In this case, fillers with hydroxyapatite filler show the maximum effect. By filling voids in tissues, the drug significantly improves skin texture, smoothes wrinkles in the selected area, and reduces wrinkles to a minimum;
- "puppet wrinkles" This is the term cosmetologists call age-related drooping of the corners of the lips. Lack of volume in this area leads to the appearance or deepening of previously formed wrinkles. Filler injections in this case give the most positive results, which remain effective for up to 2 years. The production of your own collagen in this area contributes to a significant inhibition of the aging process;
- lower jaw and chin. Age-related changes lead to the fact that over time, the skin of the face loses volume, becomes flabby, sag on the lower jaw, forming jowls. Vector lifting using filler injections can perfectly align the contour of the lower jaw and return the chin to its previous, clear contour. This type of contouring also makes it possible to get rid of a double chin;
- Hands. As old age approaches, the tendons and blood vessels on the backs of the hands become more visible, and the skin on the hands looks flabby and dry. The ideal solution in this case is the use of filler injections with calcium hydroxyapatite. This is the only drug that is approved for use in this area.
Uses [edit]
Cosmetics [edit]
Hydroxyapatite is added to some brands of cornstarch-based baby powder, such as Johnson's Aloe Vitamin E Powder. [16] According to the website, the mineral is added as an emollient to "help hydrate and soften skin." [17]
Medical[edit]
A flexible hydrogel-HA composite in which the ratio of mineral and organic substances in the matrix approaches that of human bone.
HA is increasingly used for the manufacture of materials for bone grafting, as well as for prosthetics and dental repair. Some implants, such as hip replacements, dental implants, and bone conduction implants, are coated with HA. [14] Since the natural dissolution rate of hydroxyapatite in vivo, about 10 wt.% per year, is significantly lower than the growth rate of newly formed bone tissue, when used as a bone replacement material, ways are being sought to improve its degree of solubility and thus promotes better biological activity. [18]
Hydroxyapatite is added to special toothpastes as an additive to prevent caries and reduce tooth sensitivity. [19]
Addition [edit]
Hydroxyapatite overgrows biomaterial
Microcrystalline hydroxyapatite (MCHA) is marketed as a "bone-building" supplement with superior absorption compared to calcium. [20]
This is a second generation calcium supplement derived from bovine bone. [20] In the 1980s, bone meal calcium supplements were found to contain heavy metals, [20] and although manufacturers claim their MCHA is free of contaminants, it is not recommended because its effects on the body are not good . -checked. [20]
Toothpastes with hydroxyapatite: Apagard Premio, Biorepair and Navi Maxon Sirin Doctor from Eqmaxon
Thanks to the hype created on the Cosmetist a few months ago, I suddenly discovered a new page in oral hygiene and found a new system for caring for my teeth. The hero of the occasion was hydroxyapatite
. Today I want to share some of my discoveries over these few months. If this topic interests you, I invite you to read my personal experience. First of all, I want to briefly share my “dental problems”. The enamel of my teeth is quite fragile and prone to microcracks, damage and other troubles. Therefore, I suffer from tooth sensitivity, rapid accumulation of plaque (despite regular cleaning, including professional cleaning), tartar, and cervical caries (as well as regular caries). In general, it is far from a healthy state.
Why did I point this out? To better evaluate the results of the tools, which will be discussed below. People with healthy teeth do not understand the difference when using the following products, although it is significant. The point is to change the system of approach and formula of oral hygiene products.
For about five months now I have been actively interested in this topic, since for me it has simply become a salvation and solution to many of my dental problems.
First, I actively studied all the information available to my mind about a new element for me - hydroxyapatite. First of all, I learned that:
Hydroxylapatite (hydroxylapatite)
- mineral Ca10(PO4)6(OH)2 from the apatite group, a hydroxyl analogue of fluorapatite and chlorapatite. It is the main mineral component of bones (about 50% of the total bone mass) and teeth (96% in enamel). In medicine, synthetic hydroxyapatite is used as a filler to replace parts of lost bone (in traumatology and orthopedics, hand surgery), and as a coating for implants, promoting the growth of new bone. In dentistry, hydroxyapatite is used in toothpastes as an element that remineralizes and strengthens tooth enamel.
In short, hydroxyapatite is the main element for restoring enamel. And at the same time it becomes a solution to many problems.
Thanks to the restoration and smoothness of the enamel on the teeth, less plaque and harmful bacteria accumulate, therefore the color of the teeth improves, the risk of developing caries and problems with bleeding gums decreases, fresh breath is maintained and, in general, the teeth become more protected and healthy.
Some people mistakenly believe that hydroxyapatite treats existing tooth decay, however, this is a misconception. Products containing this component help prevent
caries or help at the earliest stage, preventing caries bacteria from destroying hard tooth tissue. After all, the occurrence of dental caries is associated with a local change in pH on the surface of the tooth under dental plaque due to the fermentation of carbohydrates carried out by microorganisms and the formation of organic acids. Strengthening the hard tissues of the tooth creates barriers to these bacteria and prevents caries.
For me, the most pressing topic is desensitization. Typically, pastes to reduce this type of problem contain analgesic components that block pain. In the case of pastes with hydroxyapatite, the mechanism of action is completely different. Sensitivity is reduced by restoring enamel damage, that is, by treating rather than masking pain.
Today I’ll tell you about four toothpastes containing hydroxyapatite that I have tested in recent months. They came from three different countries. And these are not all the experimental objects with which I dealt, but I will try to talk about the rest separately if the topic is relevant and in demand.
I'll go in order of decreasing price.
Name
:
Whitening toothpaste Apagard Premio Toothpaste
Expanded opinion
:
Apagard restorative toothpastes
and
Apadent
of the Japanese company
Sangi
are based on a component such as medical nanohydroxyapatite (
nano-mHAP®
). It is a synthesized active ingredient that is close to natural hydroxyapatite and has very high compatibility with the human body.
As I understand it, it was in Japan that this component was developed and introduced into dental hygiene products. Nano-mHAP® was developed by Japanese scientists at the end of the 20th century and is recognized as an effective means of preventing and combating early caries. It is identical in composition to the very substances that make up the enamel and bone of our teeth. Due to its biocompatibility, nano-mHAP is integrated into the enamel structure, replenishing damaged areas. In 1993, nano-mHAP® was approved by the Japanese Ministry of Health as an effective anti-caries agent.
As noted on the label for the miniature toothpaste that was in my hands, Nano-mHAP®
have a triple effect:
• Replenish lost minerals in areas with incipient caries, reducing the likelihood of caries by an average of 46%.
• Fills and repairs damage to tooth enamel, making enamel strong, smooth and resistant to stains and damage.
• Effectively remove plaque and bacteria, providing optimal oral hygiene.
Apagard® Premio pastes, complementing the advantages of nano-hydroxyapatite, also include:
• Cetylpyridinium chloride
- an antiseptic that can be called universal, because both gram-positive and gram-negative bacteria, as well as various kinds of fungi and viruses, show sensitivity to it. The substance easily penetrates into tissues affected by the inflammatory process due to low surface tension. The component has a therapeutic effect not only on the surface of the mucous membranes, but also in deeper layers.
• Beta-glycyrrhetinic (glycyrrhizic) acid
– an active anti-inflammatory agent that reduces swelling of the mucous membrane and protects periodontal tissue. Glycyrrhizic acid is active against DNA and RNA viruses, including various strains of Herpes simplex, Varicella zoster, human papillomavirus, and cytomegalovirus.
The entire Apagard line of toothpastes does not contain fluoride, fluorides, parabens or artificial colors.
In general, the Japanese have done their best to make oral hygiene especially effective and safe.
Premium
Apagard
line of whitening toothpastes . Thanks to the highest content of the main active nano-component, Premio not only whitens teeth, but also restores their crystalline structure to the maximum extent possible.
The diagram below (which is shown in the Japanese version on different parts of the package, but there is also a Russian-language version on the official website) shows that it is Premio pastes that reach the maximum in several parameters when compared with other representatives of this line (Smokin' and M- Plus).
A little external description.
The blister with the miniature looks very nice, but all the information on it is conveyed in hieroglyphs, which I’m not good at.
Fortunately, on the back there is a Russian-language label with basic concepts and information.
The miniature tube, oddly enough for such a price category, was not initially sealed with foil. I hope that full-fledged products still have such protection.
The paste itself is white, quite homogeneous, slightly loose. It is distributed very well over the surface of the teeth, so you don’t need very much of it for one cleaning session (especially if you need to save on consumption). The recommendations indicate a dose of 1.0 - 1.5 cm, but I gained a little less. This was enough for an electric brush.
You should brush your teeth for 3 minutes. Swallowing is not recommended.
The paste has a neutral sweetish taste, without particularly bright mint innuendos. I like it. Of course, you won’t feel any bright freshness in your mouth after cleaning, but thanks to the quality of the paste, breathing is easy and the feeling in your mouth is very comfortable.
Foams moderately, without bubbles.
As for my personal feelings, I noticed a slight whitening (about one tone), but a couple of weeks is not enough for a full result. My dentary bone is not very amenable to intense lightening, so even this effect pleased me. In addition to a lighter tone, due to the remineralization of the enamel, the teeth feel very smooth and look shiny. Plaque does not accumulate on them.
Since the Japanese paste came to me later than everyone else, in fairness I must note that I approached it already in very good condition, since I had previously used other pastes with hydroxyapatite for several months. But there hasn’t been such a noticeable (to me) brightening effect yet, although I haven’t bought any whitening pastes.
I'm more than pleased with the result. Teeth remain smooth and clean for a long time. No inflammation, bleeding gums, or tooth sensitivity were noted.
I received the paste in one of the beauty boxes, but I took note of it and, if possible, will try to purchase either the same miniatures or a full-size tube.
Price
: 300 rubles for a blister of 20 g (as in my review), 1000 rubles for a tube of 50 g and 1550 rubles for a 100 gram tube
Testing period
: approximately 15 days (morning/evening)
Grade
: 5+ out of 5
Therapeutic and prophylactic toothpastes Biorepair
, which made so much noise here and caused a lot of controversy, are produced in Italy by the company Coswell SPA.
microRepair®
nanoparticles .
According to the manufacturer, microRepair, in terms of its chemical structure, are nanoparticles of zinc-substituted hydroxyapatite, as close as possible to the substance that makes up the hard tissues of the tooth (98% enamel and 70% dentin), which allows them to be embedded in these same enamel and dentin .
microRepair needle-shaped particles chemically bind to the hydroxyapatite of the dentinal tubules of the tooth (which house the sensory nerve endings). This ensures the elimination of sensitivity from the effects of mechanical, chemical and thermal irritants (cold and hot food).
Depending on the focus of a particular product, the percentage of particles present varies slightly.
I have already tried 4 representatives of this glorious family. There are a couple more in line. For now, I’ll tell you only about two products with which my story of admiration for hydroxyapatite began.
Name
:
Toothpaste Gum Protection Biorepair Oral Care Gum ProtectionToothpaste
Expanded opinion
:
Biorepair Gum Protection toothpaste
Designed to strengthen enamel and protect sensitive gums from inflammation.
To do this, in addition to the main active ingredient – Zinc Hydroxyapatite
(which, by the way, is in second place in the composition after water), effective components have been added:
• hyaluronic acid
, which moisturizes the mucous membrane of the gums and accelerates its regeneration;
• witch hazel and spirulina extracts
, which strengthen gums, maintain vascular tone, and prevent bleeding;
• calendula extract
, which has an antibacterial and soothing effect.
Full Ingredients
:
Aqua, Zinc Hydroxyapatite*, Glycerin, Sorbitol, Silica, Cellulose Gum, Cocamidopropyl Betaine, Aroma, Sodium Myristoyl Sarcosinate, Sodium Methyl Cocoyl Taurate, Hamamelis Virginiana Leaf Extract, Spirulina Platensis Extract, Calendula Officinalis Flower Extract, Sodium Hyaluronate, Sodium Saccharin, Phenoxyethanol, Benzyl Alcohol, Sodium Benzoate, Potassium Sorbate, Limonene, Eugenol, CI 16255
.
microRepair particle content: 18%.
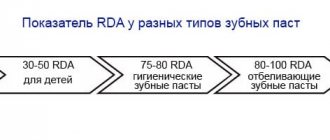
RDA (Abrasiveness Index): 14.7
Does not contain fluoride, SLS, parabens, chlorhexidine and alcohol.
According to the manufacturer, Biorepair Gum Protection toothpaste is excellent for daily use, for gingivitis, periodontitis, gum recession and tooth sensitivity, for people who have undergone oral surgery (implantation, periodontal surgery, complex tooth extraction with suturing). That is, for me (with initial gum recession, tooth sensitivity and periodic bleeding of the gums) the paste is just right. And I completely agree with this!
Instructions for use of Biorepair Gum Protection toothpaste
Squeeze the required amount of paste onto your toothbrush. Brush your teeth for 2 minutes in a circular motion, while gently massaging your gums. After brushing, it is recommended to spit out the excess toothpaste and rinse your mouth with a small amount of water. Repeat the procedure twice a day.
Personally, I used this paste only in the morning, since at the same time I used a special one at night before bed (discussed below). And the result was still excellent. With a cumulative effect.
Let's move on to the product description.
The cardboard packaging has an interesting design and a lot of information in Italian, as well as on the tube itself and in the attached paper brochure. The Russian-language label, which occupies the entire back side of the package, saves the day.
The plastic tube has a pleasant tactile sensation, since it is not smooth, but matte. These are purely personal impressions; I like these surfaces better.
The dosing hole is closed with a screw cap with a ribbed side surface, and is initially protected by a special membrane.
The paste has a pleasant pink color and a thick, dense consistency. The taste is soft, sweetish-herbal, and the smell is appropriate.
With regular use, the teeth become smooth, the amount of plaque decreases (and then stops accumulating altogether), and bleeding disappears. After a month and a half of using this paste, you can feel a big difference in the condition of the enamel coating compared to other brands of pastes. Cervical tooth sensitivity gradually decreases.
For me, the coldest months are the most critical in terms of increased pain levels, and that is when I used this paste. She helped me a lot during the most difficult periods.
I plan to repeat this paste many times, despite the rather high price.
Price
: 500-600 rubles
Testing period
: approximately 2 months (in the mornings)
Grade
: 5 out of 5
Name
:
Toothpaste Intensive Night Repair Biorepair Oral Care Intensive Night Repair Toothpaste
Expanded opinion
:
Biorepair Intensive Night Repair Toothpaste
designed to restore enamel during the night, forming a protective layer and ensuring fresh breath when waking up.
For this, in addition to the main active ingredient – Zinc Hydroxyapatite
(which is also in second place in the composition after water), the following effective components have been added:
• Zinc PCA
has an effective antibacterial effect. It eliminates the cause of unpleasant odor, providing fresh breath. Effectively fights plaque, tartar and caries, protects teeth from acid erosion.
Xylitol component
(Xylitol) also has a high antibacterial effect. Stimulates saliva production and enhances its protective properties, which is especially important for people suffering from dry mouth.
Composition of ingredients
:
Aqua, Zinc Hydroxyapatite*, Hydrated Silica, Sorbitol, Glycerin, Silica, Aroma, Cellulose Gum, Xylitol, Zinc PCA, Sodium Myristoyl Sarcosinate, Sodium Methyl Cocoyl, Taurate, Tetrapotassium Pyrophosphate, Sodium Saccharin, Zinc Citrate, Citric Acid, Ammonium Acryloyldimethyltaurate/ VP Copolymer, Benzyl Alcohol, Phenoxyethanol, Sodium Benzoate, Limonene
.
Composition contains 15% microRepair particles
RDA (Abrasiveness Index): 50
Does not contain fluoride, titanium dioxide, SLS (Sodium Lauryl Sulphate), parabens, chlorhexidine, alcohol and abrasive silica.
For this paste, the outer cardboard packaging was already Russified, so the information was all clear.
The tube is made of the same matte plastic, but in a deep blue color. And now all the words on it are again only in Italian.
The dosing hole was also pre-sealed with a special membrane. The lid is also no different, the same ribbed and unscrewing.
The paste is white, homogeneous, and easily spreads over the surface of the teeth.
The smell and taste of the night paste is more minty and invigorating (which is expected for breath freshening purposes). There is even a slight burning sensation upon application, but not too strong.
I can't say that this smell lasts until the morning. But the problem of the freshness of my breath is associated not only with the cleanliness of the oral cavity, but also with nasal congestion and the condition of the stomach. Therefore, I don’t expect any special ease in the morning. At least until I cure all the related issues. But I definitely agree that the cleanliness and comfort in the mouth upon awakening are preserved. It is very nice.
Thanks to the bactericidal properties of Zn(PCA) and xylitol, Biorepair night pastes should protect the oral cavity from the formation of tartar.
And, perhaps, the greatest value of these pastes for me personally is precisely this – in the fight against tartar. Usually, the first rudiments appear in me about a month after professional cleaning, and after a year they become very noticeable, especially in places where teeth are crowded, near cervical fillings and in areas of obvious enamel defects.
When I started using the night paste, I assumed that the manufacturers’ promises, as usual, did not quite coincide with reality. And I was so wrong!
After a month of daily use of the complex of two pastes, I noticed that the stone began to gradually disappear. After two months, only 4 lesions remained, and even then noticeably reduced. Perhaps this pleased me no less than the removal of sensitivity. I have never encountered such a result before.
At the moment, more than three months have passed since the date of professional cleaning, and not even a trace of stone formation is noticeable on all teeth, including the most problematic ones. I am delighted!
I will definitely repeat this pasta too.
Price
: 500-600 rubles (75 ml)
Testing period
: approximately 2 months (daily before bed), then occasionally
Grade
: 5 out of 5
After my first successful acquaintance with Biorepair pastes, I added to my list of purchases a paste for sensitive teeth and a special remineralizing complex with an application tray for emergency enamel restoration. And with them came two miniatures of new products that I am still planning to try. I’ll tell you the results separately if the topic is interesting and relevant.
In the meantime, I’ll move on to the most budget option on my list, which came from Korea.
Name
:
Toothpaste for sensitive teeth EQMAXON NAVI Maxon Sirin Doctor Toothpaste
Expanded opinion
:
What's so special about this
product of Korean origin?
This hydroxyapatite toothpaste is promised to effectively help remove plaque, restore enamel smoothness and strengthen teeth.
In addition to the stated hydroxyapatite
, the composition contains several more important components:
• Lactic acid
, included in the composition, perfectly reduces bleeding gums, reduces tooth sensitivity to cold, sour, hot and sweet.
• Erythritol
protects against caries and oral diseases, perfectly freshens breath.
Fluoride content: 0 ppm
Full list of ingredients
is:
D-Sorbitol Solution (70%), Tricalcium phosphate, Glycerine, Silicon Dioxide, Polyethylene Glycol 1500, Sodium Carboxymethyl Cellulose, Xylitol, Food yellow No.3, L-Menthol, Steviol Glucoside, Sodium Lauryl Sulfate, Mint Flavor NF- 1551, Methyl Parahydroxy Benzoate, Food Red No.40, Erythritol, Ascorbic acid, Tocopherol Acetate, Hydroxyapatite, Moutan Root bark Extract, Mint Flavor NF-1659, Lactic acid Bacteria Fermented Solution, Deionized Water
.
The composition is no longer as safe as that of Japanese and Italian pastes. And the substances declared as the main active ingredients are towards the end of the list. This, apparently, explains the budgetary nature of the funds.
Mode of application
: Apply a small amount of toothpaste to your toothbrush. Brush your teeth in a circular motion while gently massaging your gums. Use toothpaste daily - morning and evening after meals.
The paste was produced in Korea (by EQMAXON Corp.), so the design is not very informative, although very colorful. The Russian-language label, which, in fact, conveys the most important information, saves the day.
The dosing hole is initially sealed with a special protective film. It is very important for me. Especially for products such as hygiene products.
The lid with a dispensing hole is equipped with a snap-off plug with a convenient step for hooking and tilting. I love this design because it allows you to close the tube with one hand without dropping the squeezed-out toothpaste or brush into the sink.
The paste is pink in color, glossy, of uniform consistency, with a pleasant mint smell and a slightly more intense mint taste.
Perfectly distributed over the surface of the teeth.
The teeth feel good smoothness during application, but I am familiar with even more impressive results thanks to Biorepair and Apagard pastes. If I only used this one
paste, without comparison with analogues, the quality of cleaning and a certain reduction in sensitivity would please me compared to my previous usual options of products,
without
the use of hydroxyapatite. But knowing the best results, I was no longer so impressed with this paste.
When my husband tried this paste some time ago, he felt a huge difference in the smoothness of his teeth even after just one use. At the same time, up to
When he started testing NAVI, he also brushed his teeth with a Korean toothpaste to prevent tartar, but without hydroxyapatite.
Now he wants to use NAVI Maxon Sirin Doctor
for sensitive teeth, although he does not suffer from this problem, but he was very impressed with the quality of cleaning. Recently, my mother also joined in testing this paste. She uses it at night and is quite pleased with the result.
In this formula, the main ingredient that interests me is far from being in the forefront, so perhaps it is not as effective for my teeth as the previous options described. However, for the price, the Korean paste of large sizes and weighing 120 g is more affordable than other, more professional products with hydroxyapatite and also works well. For those who do not suffer from problems with tooth enamel like me, it is quite possible to start with a simpler option.
In my family (in two houses), two tubes of this paste are almost running out, since more people use it. And I will definitely buy it.
Price
: 175-200 rubles (for a tube weighing 120 g).
Testing period
: about 1 month constantly (morning/evening), then from time to time
Grade
: 4 out of 5
Summary
:
With the advent of pastes with hydroxyapatite in my history, a new era in oral care has begun for me. Perhaps for those who do not have dental problems like me, there is no revolutionary feeling, but there is a certain skepticism. But I do not plan to open discussions on this topic. I am simply sharing my actual results of the above products working with my actual teeth and gum problems.
As for my preferences when choosing the products that I talked about, if there are significant discounts, I definitely want to buy a full-size tube of Apagard from the Japanese founders of the topic for a full assessment of the quality of whitening. In the absence of sufficient funds in the family budget, I will buy the Korean version in order to preserve and support the overall focus of hydroxyapatite therapy. Let it be better to have little than nothing at all! But the “golden mean” (at the moment) for me is Biorepair pastes. With excellent quality of work, they are much cheaper than their Japanese counterparts. Plus, in the near future, I will apparently save money on scheduled (every six months to a year) professional cleanings.
We kindly ask you not to write harsh words and accusations in the comments that are not supported by arguments. Although personally, it was the negative reactions to reviews of Biorepair pastes that aroused my interest in the topic. And now I am completely happy. It is mine
opinion and
my
position, which, no doubt, may differ from others, but I do not impose it, but simply state the facts.
And one more huge request. If you are familiar with any other brands of toothpastes with hydroxyapatite, please share your logins and passwords! I will be very grateful!
Thanks to everyone who overcame this stream of consciousness to the end and did not lose the main thread))
Increase
Chromatography[edit]
| In this section do not cite any sources . |
The mechanism of hydroxyapatite chromatography is complex and has been described as "mixed mode". It involves ionic interactions between positively charged groups on a biomolecule (often a protein) and phosphate groups in hydroxyapatite, as well as metal chelation between calcium hydroxyapatite ions and negatively charged phosphate and/or carboxyl groups on the biomolecule. It can be difficult to predict the performance of hydroxyapatite chromatography based on the physical and chemical properties of the desired protein being purified. Elution typically uses a buffer with increasing concentrations of phosphate and/or neutral salt.
results
We showed that after combined treatment (administration of diluted CaHA and exposure to MFU-V), there was a significant increase in most indicators of cell proliferative and synthetic activity.
The combination of methods improved blood supply to tissues, which activated angiogenesis and increased the synthetic activity of cells, including the production of elastin fibers . A slight subsequent increase in the number of elastin fibers was also observed after ultrasound treatment. This may indicate that microfocused ultrasound does not damage the elastin fibers formed after the injection of filler diluted with saline, but, on the contrary, stimulates an increase in their quantity and improvement in quality. It should be noted that a study of the effect of ultrasound on skin tightening in Mongoloid patients based on skin biopsy showed an increase in the number of elastin fibers14-15.
It has been previously reported that there is an increase in the amount of type III collagen with a gradual equalization of the ratio of type I collagen2-13. In this study, we showed that the slight predominance of type III collagen was not statistically significant, since both types of collagen accumulated in almost the same ratio (0.6:0.7). The changes we identified differed from the data of previous studies, where type I collagen predominated in the facial skin after treatment. This may be due to the characteristics of the skin of the anterior abdominal wall. The skin of the anterior abdominal wall, both in terms of morphology and immunohistochemical characteristics, differs from the skin of the face. There were practically no signs of epidermal atrophy; the ratio of collagen types I and III differed, while there were more elastin fibers. These features, as well as functional differences, may explain the differences in age-related changes in these areas.
Our results are consistent with histological studies of skin treated with high-intensity focused ultrasound (HIFU) and radiofrequency waves: HIFU treatment resulted in neocollagenogenesis in the middle and deep reticular dermis and neoelastogenesis in the deep reticular dermis14-15.
The results obtained are also consistent with the histological data obtained from the clinical case reported by Casabona G et al.9-13: MFU-V treatment did not lead to a change in the histological characteristics or the effectiveness of CaHA; on the contrary, it helped to enhance the formation of new collagen and elastin fibers and improve their quality.
Use in archeology[edit]
In archaeology, hydroxyapatite from human and animal remains can be analyzed to reconstruct ancient diets, migrations, and paleoclimates. The mineral fractions of bones and teeth act as a reservoir of trace elements, including carbon, oxygen and strontium. Stable hydroxyapatite isotope analysis of humans and fauna can be used to determine whether the diet was primarily terrestrial or marine (carbon, strontium); [21] geographic origin and migratory habits of an animal or human (oxygen, strontium) [22] and reconstruct past temperatures and climate shifts (oxygen). [23] Post-depositional bone changes may promote the degradation of bone collagen, a protein required for stable isotope analysis. [24]
Data from a comparative morphological assessment of skin changes from the anterior abdominal wall
- Changes in the patients' skin after treatment were observed already at study visit M08 and reached a maximum at study visit M12 and included increased neoangiogenesis in both the superficial and deeper layers of the dermis. Van Gieson staining and Wager staining of elastin fibers showed that the accumulation of coarse collagen fibers and qualitatively changed elastin fibers continued in the extracellular matrix of the skin.
- After administration of CaHA diluted in saline, significant increases in collagen type I, collagen type III, and an endothelial marker of newly formed blood vessels were observed by immunohistochemical evaluation. These changes were enhanced after MFU-V treatment.
Defluoridation[edit]
Hydroxyapatite is a potential adsorbent for defluoridation from drinking water because it forms fluorapatite in three steps. Hydroxyapatite removes F - from water, replacing OH - forming fluorapatite. However, during the defluoridation process, hydroxyapatite dissolves and increases the pH and phosphate ion concentration, making the defluoridated water undrinkable. [25] Recently, a "calcium-corrected hydroxyapatite" defluoridation technology has been proposed to overcome phosphate leaching from hydroxyapatite. [25] This method can also affect the treatment of fluorosis by delivering calcium-fortified alkaline drinking water to fluorosis-affected areas.
conclusions
- The introduction of calcium hydroxyapatite in dilution in combination with ultrasound exposure made it possible to stimulate neoangiogenesis, enhance the synthetic activity of cells, significantly increase the number of collagen and elastin fibers and remodel both the superficial and deeper layers of the dermis.
- The procedures were well tolerated, and patient satisfaction was high.
- The results obtained indicate that the treatment with MFU-V does not disrupt, but, on the contrary, enhances the synthetic activity of cells, which makes it possible to obtain a synergistic effect when introducing CaHA to improve skin tightening.
You can learn more about the Ulthera® System at www.pro.ulthera.ru
Links[edit]
- Hydroxylapatite on Mindat
- Hydroxylapatite on Webmineral
- Anthony, John W.; Bidot, Richard A.; Bladh, Kenneth W.; Nichols, Monte S., ed. (2000). "Hydroxylapatite". Handbook of Mineralogy (PDF). IV (arsenates, phosphates, vanadates). Chantilly, Virginia, USA: Mineralogical Society of America. ISBN 978-0962209734. Archived (PDF) from the original on 09/29/2018. Retrieved August 29, 2010.
- Singh, Anamika; Tiwari, Atul; Bajpai, Jaya; Bajpai, Anil K. (2018-01-01), Tiwari, Atul (ed.), "3 - Polymer-based antimicrobial coatings as potential biomaterials: from performance to application", Handbook of Antimicrobial Coatings
, Elsevier, pp. 27– 61, DOI: 10.1016/b978-0-12-811982-2.00003-2, ISBN 978-0-12-811982-2, retrieved November 18, 2022 - Junqueira, Luis Carlos; José Carneiro (2003). Foltin, Janet; Lebowitz, Harriet; Boyle, Peter J. (Ed.). Fundamentals of Histology, text and atlas (10th ed.). McGraw-Hill Companies. paragraph 144. ISBN 978-0-07-137829-1. Inorganic matter makes up about 50% of the bone's dry weight...the crystals have defects and are not identical to the hydroxyapatite found in the rock minerals.
- Angervall, Lennart; Berger, Sven; Röckert, Hans (2009). "Microradiographic and X-ray diffraction studies of calcium in the pineal gland and intracranial tumors." Acta Pathologica et Microbiologica Scandinavica
.
44
(2): 113–119. DOI: 10.1111/j.1699-0463.1958.tb01060.x. PMID 13594470. - Ferraz, MP; Monteiro, F. J.; Manuel, C. M. (2004). "Hydroxyapatite nanoparticles: a review of production methods." Journal of Applied Biomaterials and Biomechanics: JABB
.
2
(2): 74–80. PMID 20803440. - Bouyer, E.; Gitzhofer, F.; Boulos, M. I. (2000). "Morphological study of a suspension of hydroxyapatite nanocrystals." Journal of Materials Science: Materials in Medicine
.
11
(8): 523–31. DOI: 10.1023/A: 1008918110156. PMID 15348004. S2CID 35199514. - ^ a b Ray, C.; Combs, C.; Drouet, C.; Grossin, D. (2011). "1.111 - Bioactive Ceramics: Physical Chemistry". In Ducheyne, Paul (ed.). Complex biomaterials
.
1
. Elsevier. pp. 187–281. DOI: 10.1016/B978-0-08-055294-1.00178-1. ISBN 978-0-08-055294-1. - Raynaud, S.; Champion, E.; Bernache-Assollant, D.; Thomas, P. (2002). "Calcium phosphate apatites with variable atomic ratio Ca/PI. Synthesis, characterization and thermal stability of powders." Biomaterials
.
23
(4): 1065–72. DOI: 10.1016/S0142-9612(01)00218-6. PMID 11791909. - Valletregi, M. (1997). "Synthesis and characterization of calcium-deficient apatite". Solid State Ionics
. 101–103: 1279–1285. DOI: 10.1016/S0167-2738 (97) 00213-0. - Weaver, J.C.; Milliron, GW; Miserez, A.; Evans-Lutterodt, K.; Herrera, S.; Gallana, I.; Mershon, W.J.; Swanson, B.; Zavattieri, P.; Dimasi, E.; Kisailus, D. (2012). "Club Stomatopod Dactyl: Formidable Damage-Resistant Biological Hammer". The science
.
336
(6086):1275–80. Bibcode: 2012Sci...336.1275W. DOI: 10.1126/science.1218764. PMID 22679090. S2CID 8509385. Archived from the original on September 13, 2022. Retrieved December 2, 2022. - Tanner, K. E. (2012). "Small but extremely durable." The science
.
336
(6086):1237–8. Bibcode: 2012Sci…336.1237T. DOI: 10.1126/science.1222642. PMID 22679085. S2CID 206541609. - ^ ab Habib, TU; Salisbury, H. G. (January 2022). "Biomaterials, hydroxyapatite". PMID 30020686. Archived from the original on March 28, 2022. Retrieved August 12, 2022. Quote journal requires |journal=(help)
- Carsia, C.R.; Scibek, J. S. (March 2013). "Causality and treatment of calcific tendonitis and periarthritis." Current Opinion in Rheumatology
.
25
(2): 204–9. DOI: 10.1097/bor.0b013e32835d4e85. PMID 23370373. S2CID 36809845. - "Archival copy". Archived from the original on September 13, 2022. Retrieved 25 July 2022.CS1 maint: archived copy as title (link)
- "Archival copy". Archived from the original on September 1, 2022. Retrieved 25 July 2022.CS1 maint: archived copy as title (link)
- Zhu, H.; and others. (2018). "Nanostructural insight into the dissolution behavior of Sr-doped hydroxyapatite". Journal of the European Ceramic Society
.
38
(16):5554–5562. arXiv: 1910.10610. DOI: 10.1016/j.jeurceramsoc.2018.07.056. S2CID 105932012. - Vano, M.; Derchi, G.; Barone, A.; Pinna, R.; Usai, P.; Kovani, U (January 2022). "Reduction of dentin hypersensitivity with nanohydroxyapatite toothpaste: a double-blind randomized controlled trial." Clinical Oral Research
.
22
(1): 313–320. DOI: 10.1007/s00784-017-2113-3. ISSN 1432-6981. PMID 28361171. S2CID 24712149. - ^ abcd Straub, D.A. (2007). "Calcium Supplementation in Clinical Practice: A Review of Forms, Doses, and Indications." Nutrition in Clinical Practice
.
22
(3): 286–96. DOI: 10.1177/0115426507022003286. PMID 17507729. - Richards, M.P.; Schulting, R. J.; Hedges, R. E. M. (2003). "Archaeology: the dramatic shift in diet at the beginning of the Neolithic" (PDF). Nature
.
425
(6956): 366. Bibcode: 2003Natur.425..366R. DOI: 10.1038/425366a. PMID 14508478. S2CID 4366155. Archived from the original (PDF) on March 07, 2011. Retrieved August 28, 2015. - Britton, K.; Grimes, V.; Dau, J.; Richards M.P. (2009). "Reconstructing faunal migrations using intradental sampling and strontium and oxygen isotope analyses: the case of modern caribou ( Rangifer tarandus granti
)".
Journal of Archaeological Science
.
36
(5):1163–1172. DOI: 10.1016/j.jas.2009.01.003. - Daniel Bryant, J.; Luz, B.; Froelich, P. N. (1994). "Oxygen isotope composition of fossil horse tooth phosphate as a record of continental paleoclimate". Paleogeography, paleoclimatology, paleoecology
.
107
(3–4): 303–316. Bibcode: 1994PPP…107..303D. DOI: 10.1016/0031-0182 (94) 90102-3. - Van Klinken, G. J. (1999). "Bone Collagen Quality Indicators for Paleodietary and Radiocarbon Measurements". Journal of Archaeological Science
.
26
(6):687–695. doi:10.1006/jasc.1998.0385. Archived from the original on September 13, 2022. Retrieved December 2, 2022. - ^ a b Sankannavar, Ravi; Chaudhary, Sanjeev (2019). "A Necessary Approach to Mitigate Fluorosis: Amending Aqueous Calcium to Suppress Hydroxyapatite Dissolution During Defluoridation". Journal of Environmental Management
.
245
: 230–237. DOI: 10.1016/j.jenvman.2019.05.088. PMID 31154169. Archived from the original on May 18, 2022. Retrieved June 3, 2022.
CONTENT
- 1 Chemical synthesis
- 2 hydroxyapatite with calcium deficiency
- 3 Biological function 3.1 Mantis shrimp
- 3.2 Mammal/primate/human
- 3.3 Hydroxyapatite in the remineralization of tooth enamel
- 4.1 Dentin sensitivity
Chemical synthesis[edit]
Hydroxyapatite can be synthesized by several methods such as wet chemical deposition, biomimetic precipitation, sol-gel method (wet chemical deposition) or electrodeposition. [7] A suspension of hydroxyapatite nanocrystals can be prepared by wet chemical precipitation reaction following the reaction equation below: [8]
10 Ca (OH) 2 + 6 H 3 PO 4 → Ca 10 (PO 4 ) 6 (OH) 2 + 18 H 2 O
The ability to synthetically reproduce hydroxyapatite is of invaluable clinical importance, especially in dentistry. Each method produces hydroxyapatite crystals of different characteristics, such as size and shape. [9] These changes have a marked effect on the biological and mechanical properties of the compound, and therefore these hydroxyapatite products have various clinical applications. [10]