In modern dentistry, a large number of drugs are used that help alleviate the course of a particular condition in the patient.
A special group includes drugs that are used for various forms of pulpitis to reduce pain. One of these drugs is Devit S.
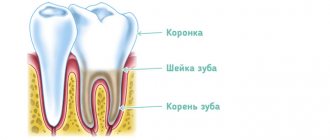
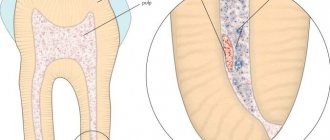
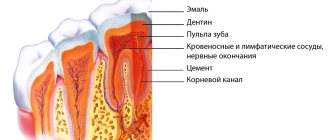
Devit S is a special paste that mummifies the pulp without using arsenic in the composition. The use of devitalizing paste promotes long-term effects on the blood vessels of the pulp without any pain or irritation.
Complete death of the neurovascular bundle occurs approximately 5-7 days after application. If the patient does not experience any discomfort, then the root canals can be filled with permanent materials within two days after using the paste.
Features of the composition
Devit S paste contains the following substances:
- paraformaldehyde, which is an antiseptic that destroys proteins and promotes devitalization of the pulp;
- lidocaine is a local anesthetic component that reduces pain that occurs during pulp mummification;
- creosote, which is an antimicrobial substance;
- paste former;
- fibrous component.
Release form: paste weighing 3 g.
Mechanism of action
The death of the pulp under the influence of paraformaldehyde occurs due to impaired tissue respiration. Enzymatic systems are destroyed, as a result of which cells cannot function normally. As a result, the pulp becomes mummified and ceases to bother the patient.
The depth of influence of a substance is determined by the duration of its use. The longer the drug is applied, the more pulp tissue will die.
An anesthetic is used to reduce pain during the necrotizing effect of the paste. In addition, it helps relieve pain from the inflammatory process in the pulp that has already begun.
Features of application
The method of using Devit S paste is as follows:
- Conducting anesthesia.
- Opening the carious cavity and completely removing necrotic dentin.
- Point opening of the pulp chamber.
- Using a thin trowel or probe, a devitalizing paste is applied to the exposed pulp horn. The approximate volume should be equal to a grain of rice. It is recommended to apply 2 times less of the drug to baby teeth than to permanent teeth.
- Then a small cotton ball is placed on the paste, which will help cushion the filling material at the site of application and thereby reduce pain.
- Finally, the carious cavity is hermetically sealed with filling material.
The first examination of the patient is scheduled after 2 days. If there is no pain, further root canal treatment is performed. If the unpleasant sensations persist, then you need to walk with the paste for another 2-3 days until the pulp completely dies.
When using pastes that do not contain arsenic, there is no need to additionally rinse the root canals with special neutralizing substances.
Devit 50,000 No. 8 tab.pp.o.
Instructions for medical use of the drug DEVIT 50,000 TRADE NAME DEVIT 50,000 INTERNATIONAL NON-PROPENTED NAME COLECALCIFEROL DOSAGE FORM FILM-COATED TABLETS 50,000 IU COMPOSITION ONE TABLET CONTAINS ACTIVE SUBSTANCE: VITAMIN D3 500 MG (50,000 IU) (EQUIVALENT TO 1.25 MG COLECALCIFEROL) , AUXILIARY SUBSTANCES: MICROCRYSTALLINE CELLULOSE PH102:290, COLLOIDAL SILICON DIOXIDE HYDRATED, DICALCIUM PHOSPHATE ANHYDROUS, SODIUM CROSCARMELLOSE, MAGNESIUM STEARATE COVER COMPOSITION: SEPIFILM LP014 TRANSPARENT (G HYDROXYPROPYL METHYL CELLULOSE, MICROCRYSTALLINE CELLULOSE, STEARIC ACID), SEPISPERS DRY 5047 RED (HYDROXYPROPYL METHYL CELLULOSE, MICRO CRYSTALLINE CELLULOSE, TITANIUM DIOXIDE E171, IRON OXIDE RED E172). DESCRIPTION FILM-COATED TABLETS, REDDISH BROWN COLOR, OVAL SHAPE. PHARMACOTHERAPEUTIC GROUP DIGESTIVE TRACT AND METABOLISM. VITAMINS. VITAMIN A AND D AND THEIR COMBINATION. VITAMIN D AND ITS DERIVATIVES. COLECALCIFEROL. ATC CODE A11CC05 PHARMACOLOGICAL PROPERTIES PHARMACOKINETICS THE PHARMACOKINETICS OF VITAMIN D HAVE BEEN WELL STUDYED. VITAMIN D IS WELL ABSORBED (IN THE SMALL INTESTINE) FROM THE GASTROINTESTINAL TRACT IN THE PRESENCE OF BILE (60-90%, IN HYPOVITAMINOSIS - ALMOST COMPLETELY). IT IS HYDROXYLATED IN THE LIVER TO FORM 25-HYDROXYCOLECALCIFEROL AND IS THEN FURTHER HYDROXYLATED IN THE KIDNEY TO FORM THE ACTIVE METABOLITE 1,25-HYDROXYCOLECALCIFEROL (CALCITRIOL). THESE METABOLITES CIRCULATE IN THE BLOOD, PLASMA AND LYMPHATIC SYSTEM BINDING WITH SPECIFIC ALPHA GLOBULIN. CIRCULATES IN THE FORM OF CHYLOMICRONS AND LIPOPROTEINS. IT ACCUMULATES IN LARGE QUANTITIES IN BONES, IN SMALLER QUANTITIES IN THE LIVER, MUSCLES, BLOOD, SMALL INTESTINE, AND IS PRESERVED ESPECIALLY FOR A LONG TIME IN ADITY TISSUE. PREPARATES INTO BREAST MILK IN SMALL AMOUNTS. VITAMIN D AND ITS METABOLITES ARE REMOVED FROM THE BODY, PRIMARILY WITH BILE AND FECES, IN SMALL AMOUNTS BY THE KIDNEYS. CUMULATES. INCREASE IN CA2+ IN THE BLOOD STARTS 12-24 HOURS AFTER TAKEN THE DRUG, THE THERAPEUTIC EFFECT IS NOTED AFTER 10-14 DAYS AND CONTINUES UP TO 6 MONTH. FEATURES OF THE PHARMACOKINETICS OF THE DRUG IN SOME GROUPS OF PATIENTS. PATIENTS WITH RENAL IMPAIRMENT EXPERIENCED A 57% REDUCTION IN THE RATE OF METABOLIC CLEARANCE COMPARED TO HEALTHY VOLUNTEERS. VOLUNTEERS WITH MALABSORPTION SYNDROME HAD A DECREASED ABSORPTION AND ACCELERATED EXCEPTION OF VITAMIN D. OBESITY VOLUNTEERS ARE LESS ABLE TO MAINTAIN VITAMIN D LEVELS WHEN EXPOSED TO THE SUN IN THE BODY, IN CONNECTION WITH WHICH THE PRESCRIPTION OF HIGHER DOSES IS REQUIRED. PHARMACODYNAMICS VITAMIN D3 REGULATES THE EXCHANGE OF CALCIUM AND PHOSPHORUS IN THE BODY, ACCELERATES THE ABSORPTION OF CALCIUM IN THE INTESTINE, IMPROVES THE REABSORPTION OF CALCIUM AND PHOSPHORUS IN THE KIDNEYS, MAINTAINS THE NECESSARY LEVEL OF THESE ELEMENTS IN THE BLOOD AND, IT PROMOTES THE FORMATION OF THE BONE SKELETON, AS WELL AS THE PRESERVATION OF BONE STRUCTURE. THE CONCENTRATION OF CALCIUM IONS IN THE BLOOD DETERMINES THE MAINTENANCE OF SKELETAL MUSCLE TONE, MYOCARDIAL FUNCTION, PROMOTES NERVOUS EXCITATION, AND REGULATES THE BLOOD CLOTTING PROCESS. ALSO VITAMIN D3 IS NECESSARY FOR THE NORMAL FUNCTIONING OF THE PARATHYROID GLANDS. WITH AGE, THE TIME EXPOSED TO THE SUN AND THE SKIN'S ABILITY TO SYNTHESIS VITAMIN D3 DECREASE. DUE TO WEAKING KIDNEY FUNCTION, THE LEVEL OF THE ACTIVE VITAMIN D METABOLITE PRODUCED IN THE KIDNEYS - 1,25(OH)2D - IS DECREASED, WHICH CONTRIBUTES TO THE WIDE PREVALENCE OF VITAMIN D DEFICIENCY AMONG ELDERLY PEOPLE. SINCE VITAMIN D IS NECESSARY FOR SUFFICIENT ABSORPTION OF CALCIUM AND NORMAL BONE METABOLISM, ITS CHRONIC DEFICIENCY CAUSES SECONDARY HYPERPARATHYROIDISM AND, AS A CONSEQUENCE, ACTIVATION OF BONE METABOLISM AND RAPID LOSS OF BONE MASS. TAKING VITAMIN D, ESPECIALLY IN COMBINATION WITH CALCIUM, LEADS TO A SLOW DOWN OF BONE LOSS AND A REDUCED INCIDENCE OF FRACTURES. VITAMIN D THERAPY IS A MANDATORY COMPONENT OF OSTEOPOROSIS PREVENTION, ALONG WITH CALCIUM Supplementation. IN ADDITION TO THIS, TAKEN VITAMIN D IS A MANDATORY COMPONENT OF COMPREHENSIVE TREATMENT OF ESTABLISHED OSTEOPOROSIS. INDICATIONS FOR USE - TREATMENT OF VITAMIN D DEFICIENCY DOSAGE AND ADMINISTRATION THE TABLET SHOULD BE SWALLOWED WHOLE (WITHOUT CHEWING) AND WITH WATER. IT IS PREFERABLE TO TAKE WITH THE MAIN MEAL. TREATMENT OF VITAMIN D DEFICIENCY IN ADULTS: 50,000 IU/WEEK (1 TABLET) FOR 6-8 WEEKS WITH SUBSEQUENT MAINTENANCE TREATMENT (EQUIVALENT OF 1400-2000 IU/DAY), I.E. 1 TABLET 50,000 IU/MONTH. MAINTENANCE THERAPY IS CONDUCT UNDER THE CONTROL OF 25-(OH)D BLOOD CONCENTRATIONS OVER THE SUBSEQUENT 3-4 MONTHS TO CONFIRM ACHIEVEMENT OF TARGET LEVELS. CERTAIN GROUPS OF PATIENTS AT HIGH RISK OF VITAMIN D DEFICIENCY MAY REQUIRE HIGHER DOSAGES AND BLOOD 25-(OH)D CONCENTRATIONS SHOULD BE MONITORED: INSTITUTIONAL OR HOSPITALIZED INDIVIDUALS LEGS · PERSONS WITH INSUFFICIENT INSOLATION DUE TO PROTECTIVE CLOTHING OR CONSTANT USE OF SUNSCREEN · INDIVIDUALS SUFFERING FROM OBESITY · INDIVIDUALS WITH A DIAGNOSED OSTEOPOROSIS · INDIVIDUALS TAKEING CONCOMITATING MEDICATIONS (E.G., ANTICONVULSAT DRUGS, GLUCOCORTICOIDS) · INDIVIDUALS A, SUFFERING WITH MALABSORPTION, INCL. Inflammatory diseases of the intestine and glutenic enteropathia · persons who undergoes vitamin D therapy, which requires supporting therapy for children, the drug is not intended for use in children and adolescents under 18 years of age. SIDE EFFECTS SIDE EFFECTS ARE USUALLY ASSOCIATED WITH EXCESSIVE USE OF COLECALCIFEROL, WHICH RESULTS IN THE DEVELOPMENT OF HYPERCALCEMIA. SYMPTOMS OF HYPERCALCEMIA MAY INCLUDE: LACK OF APPETITE, NAUSEA, STOMACH upset, WEIGHT LOSS, HEADACHES, POLYURIA, THIRST, Dizziness, CONSTIPATION, WEAKNESS, BONE PAIN, MUSCLE WEAKNESS, Stomach PAIN THOSE, MENTAL DISORDERS, DISORDERS OF RENAL FUNCTION, URINARIOUS DISEASE AND CARDIAC ARRHYTHMIA. SIDE EFFECTS ARE LISTED BY ORGAN SYSTEM CLASS AND FREQUENCY. FREQUENCY IS DEFINED AS: SOMETIMES (FROM ≥1/1,000 TO <1/100); RARE (≥1/10,000 TO <1/1,000) OR UNKNOWN (CANNOT BE ASSESSED FROM AVAILABLE INFORMATION). IMMUNE SYSTEM DISORDERS UNKNOWN: HYPERSENSITIVITY REACTIONS SUCH AS ANGIOEDEMA, LARRYNAL EDEMA METABOLISM AND NUTRITION DISORDERS SOMETIMES: HYPERCALCIAEMIA AND HYPERCALCIURIA DISORDERS OF THE SKIN AND SUBSCUTANEOUS TISSUE RARE: ITCHING, RASHE AND HURTICS CONTRAINDICATIONS - HYPERSENSITIVITY TO VITAMIN D OR COMPONENTS OF THE PREPARATION - HYPERVITAMINOSIS D — RENAL OSTEODISTROPHY WITH HYPERPHOSPHATEMIA — HYPERCALCEMIA AND/OR HYPERCALCIURIA — URINOSIS, PRESENCE OR RISK OF FORMATION OF CALCIUM KIDNEY STONES — SEVERE RENAL FAILURE — PREGNANCY — CHILDHOOD AND ADOLESCENCE UP TO 18 YEARS OF AGE WITH CAUTION: - IN PATIENTS WITH SARCOIDOSIS DRUG INTERACTIONS SIMULTANEOUS TAKING ION EXCHANGE RESINS SUCH AS CHOLESTYRAMINE, COLESTIPOL HYDROCHLORIDE, ORLISTAT, OR LAXATIVES SUCH AS PARAFFIN OIL MAY REDUCE GASTROINTESTINAL ABSORPTION OF VITAMIN D. CALCIUM ABSORPTION MAY BE REDUCED SCHENA WITH ORAL USE OF SODIUM SULPHATE OR PARENTERAL USE OF MAGNESIUM SULPHATE. CONCURRENT USE OF GLUCOCORTICOIDS MAY REDUC THE EFFECT OF VITAMIN D. CONCURRENT USE OF ANTICONVULSAT DRUGS (E.G. PHENYTOIN), HYDANTOIN, PRIMIDONE OR BARBITURATES (AND POSSIBLY OTHER DRUGS THAT CAUSE INTRODUCTION LIVER ENZYME DUTION) MAY REDUCE THE EFFECT OF VITAMIN D DUE TO METABOLIC INACTIVATION. IN CASES OF TREATMENT WITH MEDICATIONS CONTAINING DIGITALIS OR OTHER CARDIAC GLYCOSIDES, CONCURRENT USE OF VITAMIN D MAY INCREASE THE RISK OF CARDIAC GLYCOSIDE TOXICITY (ARRHYTHMIAS). STRICT MEDICAL CONTROL IS REQUIRED, AS WELL AS MONITORING OF CALCIUM CONCENTRATION IN BLOOD SERUM AND ECG, IF NECESSARY. THE CYTOTOXIC AGENT ACTINOMYCIN AND ANTI-FUNGAL AGENTS IMIDAZOLE INTERACT WITH VITAMIN D INHIBITE THE CONVERSION OF 25-HYDROXYVITAMIN D TO 1,25-DIHYDROXYVITAMIN D UNDER THE ACTION OF THE KIDNEY ENZYME, 25-HYDROXY VITAMIN D-1-HYDROXYLASE. THIAZIDE DIURETICS REDUCE URINARY CALCIUM EXCRECTION. TAKING LARGE AMOUNTS OF VITAMIN D WITH DIURETICS CAN LEAD TO EXCESS CALCIUM IN THE BODY. IN CASES OF SIMULTANEOUS TREATMENT WITH THIAZIDE DIURETICS, WHICH REDUCES URINARY CALCIUM EXCRETION, MONITORING OF CALCIUM CONCENTRATION IN BLOOD SERUM IS RECOMMENDED. PHOSPHATE SOLUTIONS SHOULD NOT BE USED TO REDUCE HYPERCALCIEMIA IN HYPERVITAMINOSIS D BECAUSE OF THE RISK OF METASTATIC CALCINOSIS. SPECIAL INSTRUCTIONS TO AVOID HYPERCALCEMIA, TREATMENT MUST BE CARRIED OUT UNDER MEDICAL SUPERVISION. WHEN CHOOSING A DOSE OF VITAMIN D, IT IS NECESSARY TO CONSIDER THE AMOUNT OF ITS CONSUMPTION FROM OTHER SOURCES (IN FOOD, dietary supplements AND FROM SUN EXPOSURE). DOSAGE IS CHOSEN INDIVIDUALLY. ENSURE SUFFICIENT FLUID INTAKE. ALL PATIENTS TAKEN PHARMACOLOGICAL DOSAGES OF VITAMIN D SHOULD HAVE REGULAR PLASMA CALCIUM CONCENTRATION CHECKED AT EACH OCCASION OF VOMITING. VITAMIN D SHOULD BE USED WITH CAUTION IN PATIENTS WITH IMPAIRED RENAL FUNCTION OR KIDNEY STONES. THERE IS NO SUFFICIENT EVIDENCE BETWEEN VITAMIN D USE AND KIDNEY STONES, BUT THERE IS A POSSIBLE RISK, ESPECIALLY WITH CALCIUM USE. CALCIUM SUPPLEMENTS SHOULD BE TAKEN UNDER MEDICAL SUPERVISION AND CALCIUM AND PHOSPHATE LEVELS SHOULD BE MONITORED. THE RISK OF SOFT TISSUE CALCINOSIS SHOULD ALSO BE CONSIDERED. USE WITH CAUTION IN PATIENTS WITH HEART DISEASE OR ARTERIOSCLEROSIS WITH A HIGH RISK OF HYPERCALCEMIA, AS WELL AS PERSONS UNDERGOING THERAPY FOR CARDIOVASCULAR DISEASES (SEE SECTION INTERACTIONS WITH OTHER DRUGS OR OTHER DRUGS HIGH FORMS OF INTERACTION – CARDIAC GLYCOSIDES, INCLUDING DIGITALIS). COLECALCIFEROL SHOULD BE PRESCRIBED WITH CAUTION IN PATIENTS WITH SARCOIDOSIS DUE TO THE RISK OF INCREASED METABOLISM OF VITAMIN D TO ITS ACTIVE FORM. IN SUCH PATIENTS, MONITORING OF CALCIUM CONTENT IN BLOOD SERUM AND URINE IS REQUIRED. THERE ARE REPORTS THAT HIGH-DOSE ORAL VITAMIN D (500,000 IU SINGLE BOLUS) RESULTS IN AN INCREASED RISK OF FRACTURES IN ELDERLY PEOPLE, WITH THE GREATEST INCREASE OCCURRED DURING THE FIRST 3 MONTHS TAKING THE DRUG. PREGNANCY AND BREAST-FEEDING LIMITED DATA AVAILABLE ON THE USE OF COLECALCIFEROL IN PREGNANT WOMEN. ANIMAL STUDIES HAVE SHOWN REPRODUCTIVE TOXICITY. THE RECOMMENDED DAILY DOSAGE FOR PREGNANT AND BREASTING WOMEN IS 400 IU, HOWEVER HIGHER DOSAGE (UP TO 2000 IU/DAY) MAY BE NECESSARY FOR WOMEN WITH VITAMIN D DEFICIENCY. DURING PREGNANCY WOMEN YOU SHOULD FOLLOW THE RECOMMENDATIONS OF YOUR DOCTOR, BECAUSE USE REQUIREMENTS DEPEND ON THE SEVERITY OF THE DISEASE AND CLINICAL RESPONSE. VITAMIN D AND ITS METABOLITES ARE EXCRETED INTO BREAST MILK; HOWEVER, THERE ARE NO CASES OF OVERDOSE CAUSED BY THE CONTENT OF VITAMIN D IN INFANTS' MILK. WHEN PRESCRIBING VITAMIN D TO A CHILD, THE DOCTOR SHOULD CONSIDER THE ADDITIONAL DOSAGE OF VITAMIN D WHICH IS TAKEN BY THE NURSING MOTHER OF THE CHILD. DURING PREGNANCY AND THE NURSING PERIOD, THE REQUIREMENTS FOR THE USE OF VITAMIN D BY WOMEN ARE LOWERED, SO DEVIT TABLETS WITH A HIGH DOSAGE OF COLECALCIFEROL 50,000 IU SHOULD NOT BE USED DURING PREGNANCY AND BREAST-FEEDING. PEDIATRICS TABLETS DEVIT 50,000 SHOULD NOT BE USED IN CHILDREN AND ADOLESCENTS UNDER 18 YEARS OF AGE. FEATURES OF THE INFLUENCE OF THE MEDICINE ON THE ABILITY TO DRIVE A VEHICLE OR POTENTIALLY DANGEROUS MECHANISMS DEVIT 50,000 DOES NOT AFFECT THE ABILITY TO DRIVE VEHICLES OR WORK WITH MECHANISMS. OVERDOSE EXCESSIVE VITAMIN D CONSUMPTION CAUSES HYPERCALCEMIA AND HYPERCALCIURIA AND MAY CAUSE THE FOLLOWING SYMPTOMS: ANOREXIA, CONSTIPATION, NAUSEA AND VOMITING, DIARRHEA, POLYURIA, DEHYDRATION, SWEATING, HEADACHE OHL, THIRSTY AND Dizziness, BONE PAIN, APATHY. A SINGLE ACUTE OVERDOSE IS NOT TOXIC AND REQUIRES MAINTENANCE TREATMENT WITH LARGE VOLUME OF FLUID ONLY. CONTINUOUS USE OF HIGH DOSES OF VITAMIN D MAY RESULT IN HYPERCALCIEMIA, HYPERCALCIURIA AND HYPERPHOSPHATEMIA. CHRONIC OVERDOSE CAN RESULT IN CALCINOSIS OF VESSELS AND ORGANS AS A RESULT OF HYPERCALCEMIA. THE CONSOLIDATED USE OF CALCIUM AND PHOSPHATES MAY RESULT IN SIMILAR DISORDERS. VITAMIN D OVERDOSE WITH HYPERCALCIEMIA IS TREATED WITH VITAMINS WITHDRAWAL, LOW CALCIUM DIET, AND AMPLE FLUIDS CONSUMPTION. IN SOME CASES, INTRAVENOUS SALT HYDRATION, SYMPTOMATIC AND SUPPORTIVE TREATMENT MAY BE REQUIRED DEPENDING ON THE PATIENT'S CONDITION. IT IS NECESSARY TO CONTROL THE CONCENTRATION OF CALCIUM IN THE PATIENT'S BLOOD AND URINE. RELEASE FORM AND PACKAGING OF 8 OR 15 TABLETS ARE PLACED IN A CONTOUR BALL PACKAGING MADE OF POLYVINYL CHLORIDE FILM AND ALUMINUM FOIL. 1 CONTOUR BALL PACKAGING TOGETHER WITH INSTRUCTIONS FOR MEDICAL USE IN THE STATE AND RUSSIAN LANGUAGES IS PLACED IN A CARDBOARD PACK. STORAGE CONDITIONS STORE IN A PLACE PROTECTED FROM MOISTURE AT A TEMPERATURE NOT ABOVE +30°C. KEEP OUT OF THE REACH OF CHILDREN. SHELF LIFE: 3 YEARS DO NOT USE AFTER EXPIRATION. TERMS OF DISCHARGE FROM PHARMACIES WITHOUT A PRESCRIPTION Manufacturer Quest Vitamins Middle East Physics, UAE Dubai, UAE, Jebel Ali Free Zone, Plot S20708A Registration Certificate Holder Lamira LLP, 3rd Floor, 49 Farrington Road, London EC1M 3JP, UK Name, address and contact details (telephone, fax, e-mail) of the organization on the territory of the Republic of Kazakhstan, accepting claims (suggestions) on the quality of medicines from consumers, responsible for post-registration monitoring of the safety of the medicine: ALDIMED LLP 050051 Republic of Kazakhstan, Almaty, microdistrict . Samal 1, building 1 Tel.; Email address
Precautionary measures
The patient must be warned that if any uncharacteristic taste appears in the oral cavity, it is necessary to immediately consult a doctor, as there is a possibility of devitalizing paste leaking through the filling.
If the permissible period of action of the drug is exceeded, local toxic effects may develop. The gums around the tooth change its color, and the pain changes its character. Severe discomfort appears when biting, which indicates the progression of toxic periodontitis.
Technical features of applying paste
The operation to devitalize the dental cavity consists of applying a paste containing arsenic to the open horn of the pulp, which is collected on the tip of the probe. This volume is approximately 40 times less than a pinhead, based on the calculation that the maximum single dose is 3 mg and the maximum daily dose is 10 mg.
After application, the devitalizing paste is covered (without applying pressure) with a piece of cotton wool soaked in an anesthetic solution. The dental cavity is covered with a loose bandage of water dentin, which has a temporary purpose, but ensures absolute tightness of the dental cavity. Taking into account the size of the pulp chamber (depending on the number of tooth roots), the composition is removed from the cavity after 36 or 24 hours, but if chemical periodontitis occurs, the procedure is performed immediately.
Adverse reactions
If the drug is used carelessly and incorrectly, the following complications may develop:
- destruction of bone tissue;
- damage to the jaw nerves;
- burns of the oral cavity;
- necrotic changes in the gingival papillae;
- constant pain, which only intensified after using the paste;
- When treating baby teeth, there is a risk of damage to the permanent tooth germ.
If such phenomena occur, it is necessary to immediately remove the temporary filling and remove the drug. The root canals are washed with antidite and antiseptics. The mucous membrane is treated with healing substances.
Possible reactive pain
The patient is warned in advance about the likelihood of developing short-term (within 2 hours) reactive pain, and is offered to mitigate them with the help of Amidopyrine or bromine preparations.
In addition to quickly passing reactive pain, the use of pastes with arsenic acid compounds threatens the occurrence of severe chemical burns when it is washed out or the cavity is insufficiently sealed, up to necrosis (osteomyelitis). Another consequence of the use of arsenic pastes is the appearance of persistent toxic periodontitis, which is often observed with prolonged and excessive exposure to arsenic.
Thus, in order to avoid damage to the periodontium with the development of foci of necrosis during the use of arsenic, when calculating the dosage and exposure time, the dentist must evaluate such factors as the patient’s age and tooth weight.
Many people are interested in what the effect of devitalizing paste is. Scientific research has proven that the use of adequate dosages of arsenous acid, due to its diffusion into the periapical space, provokes not only the destruction of the dental pulp, but also stimulation of its stump, and also leads to the regeneration of periodontal tissue.
Cost and analogues
The price of Devit S ranges from 100 to 130 rubles per syringe; analogues are also available for purchase:
- Depulpin . Produced in Germany.
- Caustinerf forte . Produced in France.
- Non-arsenic . Produced in Russia.
- Devitec AS . In production.
- Devital . Produced in Russia.
- Devit ARS . Arsenic-based analogue. The disadvantages of its use are such as high toxicity and the impossibility of using it in the treatment of baby teeth. Also produced.
- Devit P. Used in children. Instead of creosote, the composition contains camphor, menthol and chlorophenol.
All analogues presented have a higher cost, although their effectiveness is the same as that of Devit S.
Devitalizing paste "Devit"
As noted above, this brand of paste comes in three types and is used in various dental methods of dental therapy. It is used for pulp devitalization in the treatment of pulpitis during mortal amputation or extirpation, for the treatment of residual pulpitis, and is also used as an additional means for devitalization during repeated procedures after using devitalizing pastes containing arsenic.