Lidocaine is an effective pain reliever. Local anesthetics inhibit the ionic currents involved in the formation of the irritant, due to which the analgesic effect is achieved. Lidocaine stabilizes neuronal membranes, suppresses nerve conduction, and reduces the degree of depolarization.
When is lidocaine indicated?
The range of indications for Lidocaine solution includes the following conditions:
- Local anesthesia required for manipulations in ophthalmology, ENT practice, dentistry, and surgery.
- The use of cephalosporin group of antibacterial drugs as a solvent, the administration of which is characterized by a pronounced pain effect.
Lidocaine for injection allows you to anesthetize the desired area of skin or mucous membrane for several hours, which will be enough for medical manipulation accompanied by increased sensitivity.
Where is topical anesthesia used?
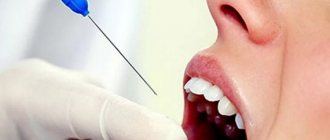
Most often it is used for pain relief when puncturing the skin during catheter insertion, injections, blood sampling, as well as in the following areas of medicine, such as:
- dentistry (for removing teeth and tartar, to relieve the gag reflex during procedures);
- surgery (for superficial surgical interventions. For example, when harvesting a flap for skin grafting, during mechanical cleaning of trophic ulcers);
- dermatology (for removal of molluscum contagiosum);
- cosmetology (before tattooing, hair removal, mesotherapy, laser therapy);
- gynecology (for removal of condylomas);
- pediatrics (for blood sampling, vaccinations).
How is lidocaine solution used?
The local anesthetic solution is pre-tested on a separate area of the skin, which makes it possible to assess the presence or absence of an individual negative reaction to the drug.
The dosage of lidocaine solution depends on the type of anesthesia:
- Terminal type of anesthesia involves local application of a solution (2 mg per kg of body weight), maximum 20 ml for an adult patient. The effect lasts no more than 30 minutes.
- Conduction type of anesthesia. The dosage depends on the size of the area that needs to be anesthetized. Maximum dose 20 ml.
- In ophthalmology, the solution is instilled into the conjunctival sac.
- Epidural anesthesia – 22-30 ml of solution.
- Pain relief in obstetrics and gynecology – 20-30 ml of solution.
- In childhood, the dosage is determined individually (on average 3.5-4 mg per kg of weight).
To prevent the development of side effects, the solution must be administered strictly in recommended dosages. The medication Lidocaine, the instructions for use of which contains all the necessary dosages, must be prescribed after a detailed study of the anamnesis.
Lidocaine spray and EMLA: what is the difference?
The drugs differ in composition. If the spray contains only one substance - lidocaine, then EMLA cream contains lidocaine along with prilocaine.
Lidocaine and prilocaine belong to the class of amides of intermediate action. Their effect lasts on average 1-1.5 hours.
The anesthetic effect of lidocaine is 4 times higher than that of novocaine. It does not penetrate well through the surface epithelium of the skin. Therefore, the spray is more often used when performing manipulations on the mucous membranes - there the effectiveness of pain relief is higher.
Prilocaine is slower but longer acting than lidocaine and is less toxic. The combination of two anesthetics in EMLA cream allows you to prolong the effect of the drug and provide it with additional functions. For example, with ELMA the depth of pain relief depends on the duration of application, while with lidocaine spray the effect extends only to the superficial layers of the skin and then only when additional conditions are created (applying a patch or film that prevents moisture evaporation). Thus, the cream is suitable for both procedures performed on the skin and those performed on the mucous membranes.
Adverse reactions
If used incorrectly, adverse reactions may occur:
- Dizziness, drowsiness, convulsions, sensory disturbances.
- Tinnitus.
- Changes in blood pressure, cardiac dysfunction.
- Nausea, vomiting.
- Feeling of heat, swelling at the injection site.
- Burning sensation, thrombophlebitis.
The list of adverse reactions is much wider, so it is necessary to monitor the patient’s condition after administration of the lidocaine solution.
Contraindications to the use of lidocaine solution
The use of the drug is prohibited in patients with individual intolerance to the active substance. Lidocaine injections are also prohibited for use in the following conditions:
- The first few months after myocardial infarction.
- Severe cardiac pathologies with bradycardia.
- Infectious process at the injection site.
- Period of pregnancy, lactation.
- Children's age (up to 15 years).
- Convulsions due to epilepsy.
In these conditions, the use of lidocaine solution is not recommended.
Lidocaine spray 10%, 38g
Registration Certificate Holder
EGIS Pharmaceuticals (Hungary)
Dosage form
Medicine - Lidocaine
Description
Spray for topical use, dosed
in the form of an almost colorless alcohol solution with a characteristic menthol odor.
1 dose
1 vial.
lidocaine 4.6 mg 3.8 g
Excipients
: peppermint leaf oil, propylene glycol, ethanol 96%.
38 g (650 doses) - dark glass bottles with a capacity of 50 ml (1) with a dosing pump with a spray head - cardboard packs.
Indications
For terminal (superficial) anesthesia of mucous membranes: in dentistry, in otorhinolaryngology, in obstetrics and gynecology, for instrumental and endoscopic examinations, in surgery and dermatology, radiographic examination (elimination of nausea and pharyngeal reflex).
Contraindications for use
Use for tonsillectomy and adenotomy in children under 8 years of age;
hypersensitivity to lidocaine. With caution
during instrumental studies (rectoscopy) in patients with hemorrhoidal bleeding, local infection in the area of application, trauma to the mucous membrane or skin in the area of application, severe somatic pathology, epilepsy, bradycardia, cardiac conduction disorders, impaired liver function, severe shock, young children, elderly patients, during pregnancy and lactation.
pharmachologic effect
Local anesthetic for superficial anesthesia. The action is due to the blockade of voltage-dependent sodium channels, which prevents the generation of impulses at the endings of sensory nerves and the conduction of pain impulses along nerve fibers.
When applied topically, it dilates blood vessels and does not have a local irritating effect. Has an analgesic effect.
The effect develops 1-5 minutes after application to mucous membranes or skin and lasts 30-60 minutes.
Drug interactions
Cimetidine and propranolol reduce the hepatic clearance of lidocaine (decreased metabolism due to inhibition of microsomal oxidation and decreased hepatic blood flow) and increase the risk of toxic effects (including stunned state, drowsiness, bradycardia, paresthesia).
Barbiturates, phenytoin, rifampicin (inducers of microsomal liver enzymes) reduce effectiveness (an increase in dose may be required).
When prescribed with ajmaline, phenytoin, verapamil, quinidine, amiodarone, the negative inotropic effect may be enhanced. Co-administration with beta-blockers increases the risk of bradycardia.
Curare-like drugs enhance muscle relaxation.
Procainamide increases the risk of developing central nervous system excitation and hallucinations.
With the simultaneous administration of lidocaine and hypnotics and sedatives, their inhibitory effect on the central nervous system may be enhanced.
With intravenous administration of hexobarbital or sodium thiopental against the background of the action of lidocaine, respiratory depression is possible.
Under the influence of MAO inhibitors, the local anesthetic effect of lidocaine may be enhanced.
With the simultaneous use of lidocaine and polymyxin B, an increased inhibitory effect on neuromuscular transmission is possible, so in this case it is necessary to monitor the patient's respiratory function.
Dosage regimen
Apply locally, externally. The dose depends on the indications and the area of the anesthetized surface.
Side effect
Local reactions:
a slight tingling sensation that disappears as the anesthetic effect develops (within 1 minute); Transient erythema, swelling and sensory disturbances may occur.
Allergic reactions:
very rarely - urticaria, angioedema, bronchospasm; in exceptional cases - anaphylactic shock. The use of the drug should be stopped immediately if any allergic reaction occurs.
After topical application, systemic effects rarely develop, because
Only a very small amount of the active substance can enter the bloodstream. From the side of the central nervous system:
very rarely - nervous excitement, systemic dizziness, insomnia, loss of consciousness and respiratory paralysis.
From the cardiovascular system:
decreased blood pressure, depression of myocardial function, bradycardia, cardiac arrest.
special instructions
Use with caution in patients with epilepsy, as well as bradycardia, cardiac conduction disturbances, impaired liver function and severe shock, especially when significant amounts of the drug can be expected to be absorbed when large areas of tissue are treated with high doses.
It is important to prevent lidocaine from entering the respiratory tract (risk of aspiration).
Application to the buccal mucosa is associated with a risk of dysphagia and subsequent aspiration, especially in children. If the sensitivity of the tongue and mucous membrane of the cheeks is impaired, the risk of biting them increases.
Lidocaine is well absorbed through mucous membranes (especially in the trachea) and damaged skin. This should be taken into account, especially when treating large areas of tissue in children.
In cases of use during surgical operations in the pharynx or nasopharynx, it should be taken into account that lidocaine, suppressing the pharyngeal reflex, enters the larynx and trachea and suppresses the cough reflex, which can lead to bronchopneumonia. This is especially important in children, as they are more likely to trigger their swallowing reflex. In this regard, the spray is not recommended for local anesthesia before tonsillectomy and adenotomy in children under 8 years of age.
Caution should be exercised when applying lidocaine to damaged mucous membranes and/or infected areas.
Lower doses should be used in weakened and elderly patients, in acute diseases, as well as in children - in accordance with age and general condition.
Effect on the ability to drive vehicles and machinery
During the treatment period, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Use during pregnancy and breastfeeding
Restrictions during pregnancy - With caution. Restrictions when breastfeeding - With caution.
If local anesthesia is necessary and no safer treatment is available, lidocaine can be used during pregnancy.
Lidocaine is excreted in breast milk, but after topical use in normal therapeutic doses, the amount excreted in milk is too small to cause any harm to a nursing infant.
Use for renal impairment
Restrictions for impaired renal function - With caution.
In chronic renal failure, accumulation of metabolites is possible.
Use for liver dysfunction
Restrictions for liver dysfunction - With caution. The drug should be used with caution if liver function is impaired.
Use in elderly patients
Restrictions for elderly patients - Use with caution.
Use with caution in elderly patients.
Use in children
Restrictions for children - Use with caution. Use with caution in children. Use for local anesthesia before tonsillectomy and adenotomy in children under 8 years of age is not recommended.
Interaction of the solution with other drugs
Lidocaine is not recommended to be combined with the following groups of drugs at the same time:
- Analgesics of the narcotic group - threatens respiratory depression.
- Anticoagulants – increases the risk of bleeding.
- Cardiac glycosides – their effectiveness decreases.
- Novocaine group – increases muscle relaxation.
- The standard is a negative effect on respiratory function.
Lidocaine solution should be combined with extreme caution in diseases of the heart and nervous system, accompanied by systematic use of drugs.
special instructions
Lidocaine injection should only be done under the supervision of a physician. Treatment should be carried out under ECG control.
Before starting treatment, it is recommended to examine the level of potassium in the blood, since if its content is reduced, the effectiveness of the drug decreases.
The drug has an effect on the central nervous system, so it is not recommended to drive or engage in potentially life-threatening activities while using it.
Careful monitoring is required for patients prone to seizures, as even small doses may enhance the convulsive effect.
It is necessary to combine lidocaine with caution with those drugs that increase its bioavailability or slow down its elimination, which can be especially dangerous in case of end-stage renal failure.
With intramuscular administration of lidocaine solution, increased activity of creatine phosphokinase may be observed, which prevents the diagnosis of myocardial infarction.
Rapid administration of lidocaine solution can lead to a sharp decrease in blood pressure, with the possibility of collapse. In this case, the toxic effect of the drug with its cardiotoxic effect should also be considered.
Possible side effects
Like all medicines, lidocaine can cause side effects, although not everyone gets them.
Severe allergic reactions (rare, may affect 1 in 1,000 people)
If you have a severe allergic reaction, tell your doctor immediately.
Its symptoms may include sudden onset:
- Swelling of the face, lips, tongue, or throat. This may cause difficulty swallowing.
- Severe or sudden swelling of the arms, legs, and ankles.
- Difficulty breathing.
- Severe itching of the skin (with swelling).
Other side effects:
- skin irritation in areas where lidocaine is used,
- nervousness,
- dizziness,
- drowsiness,
- loss of consciousness,
- a sore throat,
- hoarse voice or loss of voice,
- lowering blood pressure. This may lead to dizziness or delirium,
- convulsions,
- difficulty or slow breathing,
- bradycardia,
- stopping breathing or heartbeat.
Side Effect Reporting:
If you notice any side effects, tell your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects by going to the website www.arpimed.com and filling out the appropriate form “Report a side effect or ineffectiveness of a drug” and to the Scientific Center for Expertise of Medicines and Medical Technologies named after. Academician E. Gabrielyan by going to the website www.pharm.am to the “Report a side effect of a drug” section and fill out the form “Card of reporting a side effect of a drug.” Scientific center hotline phone number: +37410237665; +37498773368
How to store Lidocaine
- Keep out of the reach of children.
- Shelf life – 3 years. Do not take Lidocaine after the expiration date indicated on the drug package. When indicating the expiration date, we mean the last day of the specified month.
- Store out of reach of children, protected from light at a temperature not exceeding 150C.
- Your doctor/dentist or other healthcare provider should ensure that lidocaine is stored properly. Appropriate hospital personnel are responsible for the proper storage, administration, and disposal of lidocaine.
- Medicines should not be disposed of in wastewater or sewer systems. Ask your pharmacist how to dispose of any medicine you no longer need. These measures are aimed at protecting the environment.
Package contents and additional information
What Lidocaine contains
active substance: lidocaine – 50 mg;
other components: light liquid paraffin, isopropyl myristate, propylparaben.
What Lidocaine looks like and contents of the pack
Transparent colorless solution.
Description of packaging
primary: 15 ml of a 5% solution (spray) is filled into a plastic bottle with a metered mechanical sprayer.
secondary: 1 bottle along with an insert leaflet is placed in a cardboard pack.
Vacation conditions
Dispensed by prescription.