Author of the article:
Soldatova Lyudmila Nikolaevna
Candidate of Medical Sciences, Professor of the Department of Clinical Dentistry of the St. Petersburg Medical and Social Institute, Chief Physician of the Alfa-Dent Dental Clinic, St. Petersburg
For many years, every second person in the street was convinced that a good toothpaste must certainly contain fluoride. Our grandparents stubbornly insisted on the importance of this element. But is fluoride really necessary in toothpaste, or does the harm of this microelement still outweigh its benefits? Let's try to figure it out.
The history of fluoride in toothpastes
Fluoride was first used in toothpastes more than 100 years ago, in 1914 in the USA. At the beginning of the 20th century, scientists found that this element is an excellent protection against caries and its use reduces the rate of tooth decay by an average of 30-45%. Under the influence of fluorine, enamel became resistant to acids and more durable.
Why is fluoride needed in toothpaste?
Surprisingly, the same element can be both healing and destructive to the body. And fluoride is just that: the substance can both restore and strengthen tooth enamel, and cause irreparable harm to it. It is thanks to fluoride that the strength of bone tissue in the body is maintained; this element plays a key role for the health of hair, nails and teeth. Fluoride is very important in the growth and development of a child: without it, his skeleton does not develop normally.
In addition, fluorine has a beneficial effect on metabolism: without this element, the body cannot remove heavy metals. The substance also helps absorb iron and supports immunity.
When there is a lack of fluoride in the body, bones and teeth are the first to suffer. Bones bend, become brittle and brittle, and in the event of a fracture they heal very slowly. With a lack of fluoride, the enamel gradually becomes thinner, it is increasingly affected by plaque bacteria, and caries develops faster and faster.
In fact, it is not difficult to provide the body with its daily requirement of fluoride. Products high in this substance include:
- a variety of fruits, such as grapefruits and apples;
- nuts;
- dairy products;
- various types of meat (especially liver);
- various vegetables - pumpkin, spinach, potatoes;
- buckwheat and oatmeal porridge;
- honey;
- any types of tea.
By drinking enough water per day and eating right, you can provide yourself with fluoride. Therefore, the need for toothpastes with fluoride should only be determined by a dentist. As a rule, fluoride products are prescribed to smokers and people who abuse coffee.
Relevance of the issue
The human body cannot fully exist and function normally if there is no source of fluoride, for example, sodium monofluorophosphate. Chemists and doctors, especially dentists, know well what this compound is. Fluorine is an extremely important trace element for the human body, which is usually present in the form of compounds. The main percentage of flora accumulates in dentin, covering the dentition, enamel, and bone tissue. About 0.03 mg per day per kilogram of weight for an adult must be obtained from external sources. For a child, indicators vary between 0.15-0.1 mg/kg.
In medicine, the substance is classified as an inhibitor of bone resorption and a caries preventive agent. If necessary, sodium monofluorophosphate is indicated to replenish fluoride deficiency. Chemists can explain what it is: the substance is a powder formed by colorless crystals. To dissolve, you need to take 25 times more water than the compound is available.
Pros of fluoride toothpastes
Many people specifically choose hygiene products with fluoride to strengthen their teeth and prevent the development of caries. Indeed, in some cases, this element protects the enamel from the action of harmful bacteria and strengthens it. Under the influence of fluoride, less acid is released, which affects the enamel. Therefore, anti-caries toothpastes often contain fluoride.
The main benefits of fluoride toothpastes include:
- antiseptic effect;
- improvement of metabolism;
- improvement of the remineralizing effect of saliva;
- stimulation of the salivary gland;
- inhibition of the transformation of soft plaque into tartar.
Is fluoride harmful or beneficial?
So, fluoride is an actively used component in most toothpastes. This substance reduces the likelihood of developing caries and strengthens the enamel. Complete refusal from at least periodic use of fluoride-containing toothpastes increases the rate of caries development by about a third and contributes to the development of tartar and plaque.
The most effective compounds containing fluoride are amino fluoride and sodium fluoride, therefore, when choosing a paste in the store, you should carefully look at what exactly is included in its composition.
During cleaning, fluoride, unless, of course, the paste is intentionally swallowed, does not enter the bloodstream.
Fluoride-containing pastes should not be used:
- young children, as they often swallow the paste;
- residents of regions where the amount of fluoride in water significantly exceeds the norm (for example, this is observed in Odintsovo, Krasnogorsk, Tver, Serdobsk, Yegoryevsk and other settlements).
For other categories of people, fluoride does not pose any threat.
Sometimes you can hear opinions that fluoride is not just harmful, but even dangerous. The only harm that can be caused by excess fluoride is provoking the development of fluorosis, and even then the disease affects exclusively children whose teeth have not yet begun to erupt. There are no proven cases of the development of fluorosis from the use of pastes: the disease is detected only in children living in regions with the amount of fluoride in drinking water above 1 mg per liter.
You can find many articles online, the authors of which claim that fluoride is extremely dangerous. However, there is no evidence of this.
Harm of toothpastes with fluoride
Unfortunately, many of us forget that toothpaste is not the only source of fluoride. This element is found in many foods and even in ordinary tap water.
Before you buy even the best toothpaste with fluoride, it is important to understand your need for this element: its daily intake should not exceed three milligrams. In order to get this dose, it is enough to drink two liters of water a day.
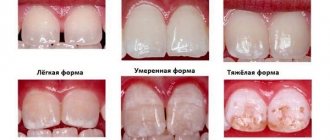
For a person who eats right and drinks enough water, it is not at all necessary, and often even harmful, to use toothpastes with fluoride. The fact is that this element in excess amounts is toxic to the body. The substance accumulates in tissues, including tooth enamel. If too much fluoride accumulates in it, fluorosis appears, the process of destruction of enamel.
Fluorosis is very easy to recognize. The disease manifests itself as white spots on the surfaces of the teeth. These spots grow quickly, turn yellow and begin to corrode the enamel. In case of fluorosis, it is important to start treatment as soon as possible - remineralizing therapy, photophoresis or the use of special applications.
Often, teeth with fluorosis require additional whitening, because it is almost impossible to cope with such a problem with the help of professional cleaning.
The effect of fluoride on teeth
What does fluorine have to do with it? The answer is simple: the effect of fluoride on teeth is realized precisely through these processes.
Fluorine, or more correctly fluoride, can be stable or labile.
Stable fluoride is one that is included in the hydroxyapatite crystal lattice instead of the OH group. This happens both before the tooth erupts and after. A new compound is formed - fluorapatite. Fluorapatite is more stable, resistant to acid (its dissolution begins at pH = 4.5), less soluble (it precipitates more easily than hydroxyapatite).
Everything would be fine, but in the conditions of the oral cavity, fluoride in the crystal lattice of enamel does not change its properties so much. The fact is that the enamel contains many crystals with inclusions of CO3 groups - biological carbonapatites. The effect of fluorine on carbonapatite is not as pronounced.
Therefore, today it is believed that the main protective mission is carried out by another fluoride - labile . This is fluoride that is found near the surface of the enamel. He has several sources:
— dissolving stable fluoride;
- dissolving calcium fluoride. It is formed simultaneously with fluorapatite if there is a lot of fluorine for a long time in an acidified environment (there are more calcium ions);
- from intercrystalline enamel fluid;
- from dental plaque and oral fluid.
Labile fluoride affects:
- Demineralization;
- Remineralization, including during initial caries, stabilizing it;
- Activity of dental plaque.
Let's take a closer look at each of the points:
- Labile fluoride prevents demineralization. It maintains the right amount of fluoride ions, and the enamel fluorapatite does not need to dissolve to replenish this amount.
- Labile fluoride helps remineralization. This happens due to several effects of fluoride:
Firstly, the precipitation of calcium and phosphate ions is facilitated. It creates this opportunity under much less favorable conditions (more acidic environment and fewer ions). Fluoride binds them, forming fluorapatite and calcium fluoride. When deposited, these minerals, on the one hand, block the entrances for acid into the enamel, and on the other hand, they interfere with the removal of calcium and phosphates from the enamel. It is also a good supply of ions for the inner layers of the crystal lattice.
Secondly, fluoride accelerates the growth of enamel apatite crystals.
Thirdly, fluorine penetrates more easily and quickly between enamel crystals in the composition of HF acid. It is then released from the acid and repairs and strengthens the crystals themselves.
There is one interesting point in the effect of fluoride on a carious lesion. It is because of it that a lesion characteristic of caries is formed with a harder surface layer (1) and a demineralized subsurface (2) even at very low concentrations. There are many opinions about why this happens. In any case, a dentist in a clinic should always remember this feature when examining teeth and diagnosing caries.
- Labile fluoride affects the activity of dental plaque.
In dental plaque, fluoride can be found both outside and inside the microbial cell. As the plaque environment becomes more acidic, more HF acid is formed. This acid easily penetrates the microbial cell. The H+ ion acidifies its cytoplasm, and the F– ion has the following effect:
- Reduces the activity of enolase, an enzyme in the glycolysis reaction, the final product of which is destructive lactic acid;
- Reduces ATP formation. And this is the “key” of cellular metabolism, growth, and a necessary element for the operation of the enzyme that transports glucose into the cell - a source for acid production, and the H + /ATPase enzyme, which maintains the necessary pH inside the cell. Plus, thanks to the work of this same H+/ATPase, the energy that is needed for the life of the microorganism is generated. The enzyme does not work - there is no energy for the cell.
- Fluoride inhibits the microbial cell's synthesis of the macromolecules it needs - proteins, carbohydrates, including extracellular ones - levans, glucans. Levan and glucan are produced by Str. mutans to organize, unite dental plaque (to make it easier for other bacteria to attach and live on the tooth).
If you go beyond the microbial cell, fluoride reduces the growth rate of dental plaque and reduces the content of Str.mutans - the main culprit of caries. It is more sensitive to fluoride than other microorganisms, and the pH of dental plaque in the presence of fluoride is not low enough for it to live comfortably and produce acid.
But outside the laboratory, in vivo, the effects of fluoride on dental plaque are controversial:
- A lot of fluoride is needed to achieve a bactericidal concentration;
- Microorganisms adapt to dangerous levels of fluoride.
Fluoride has a more pronounced effect directly on dental plaque due to other ions with which it is combined in preventive preparations.
Indications for the use of fluoride pastes
Of course, in some cases, toothpastes with fluoride will be useful to patients, but, as we have already said, only a dentist should prescribe them. The specialist will assess the condition of the enamel and oral cavity, and then determine whether the teeth have enough fluoride and what kind of toothpaste they need.
Therapeutic toothpastes with calcium and fluoride are indicated for use if the patient has:
- signs of dental demineralization;
- poor enamel resistance to acids;
- fragility of teeth, tendency to chip;
- minor signs of caries.
The fluorides contained in such toothpastes create a thin protective layer on the crowns. All substances hazardous to enamel do not penetrate it and their negative impact is reduced several times. Incoming fluoride, when it is deficient in the body, slows down the proliferation of bacteria and, accordingly, the development of caries. The likelihood of gum inflammation is also reduced.
It is forbidden!
Sodium monofluorophosphate will become a source of harm if it is used by a child under six months of age. For systemic use, fluorides are prohibited if a person has kidney or liver diseases and dysfunction of these systems. You should not take fluoride in food if a peptic ulcer localized in the intestines or stomach has worsened. The period of childbearing and lactation also becomes a limitation. Fluoride should be used with caution in children under fourteen years of age.
Consequences of excess fluoride in the body
Do not forget that fluorine is, first of all, a toxic substance. It is added to toothpastes only in the form of compounds that can release active ions. The pastes contain sodium fluoride, sodium monophosphate, as well as tin and aluminum fluorides, and amino fluoride.
When fluoride enters the body of an adult in excess quantities, the following problems may begin:
- disruption of metabolic processes and, as a result, destruction of bones and teeth;
- disruptions in the functioning of the endocrine system;
- liver and kidney diseases;
- increased blood pressure;
- decreased immunity.
Surprisingly, translated from Greek, the word fluorine means “destroying.” Fluorine compounds in the gaseous state are deadly. And fluorites, often added to toothpastes, are considered one of the most harmful substances for the body.
If you use fluoride paste constantly, without apparent need, the following changes may occur in the oral cavity:
- damage to the enamel, the appearance of stains on it;
- fragility of teeth;
- increased sensitivity of teeth.
When is it used?
It is common to use fluoride, which produces sodium monofluorophosphate, as an element of a therapeutic course for osteoporosis. The substance is recommended both for people suffering from the steroid type and for those for whom the risks of such a pathology are assessed above average. Fluoride compounds can be taken by patients with primary osteoporosis of various natures, including the idiopathic form, in which the etiology of the case cannot be established. Fluoride compounds are prescribed to prevent caries in people of any age, as well as as part of a therapeutic course for local osteopathy. Fluoride supplements are recommended if the water a person drinks contains less than 0.6 mg/ml fluoride.
Children's toothpastes with fluoride
Children's toothpastes should absolutely not contain fluoride, despite the fact that this element is necessary for growing organisms. Fluoride is added only to toothpastes for children and adolescents over 5 years of age. The thing is that young organisms perceive various components much more strongly and are many times more susceptible to fluorosis (excess fluoride).
So, now you know the benefits and harms of fluoride in toothpastes. You should not decide on the use of such means on your own. Only an experienced dentist should prescribe pastes with fluoride and calcium.
To avoid the need for medicinal pastes, take good care of your teeth and be attentive to your body. Do not get carried away with coloring products, coffee, tea and sweets. For dental health, the absence of bad habits, proper, balanced nutrition and daily thorough hygiene procedures are important.
An excellent tooth support product is ASEPTA “Remineralization” professional toothpaste with high concentrations of hydroxyapatite and thermal mud. This remedy restores tooth enamel, reduces the sensitivity of teeth and gums and accelerates the regeneration of the oral mucosa.
Features of different forms of sodium
If you examine the compositions of modern toothpastes, you will notice that they contain various components containing sodium. Their benefits for human health differ. For example, sodium monofluorophosphate, as it became clear during experiments, decomposes quite slowly. Doctors recommend brushing your teeth for no more than a couple of minutes, and this time is usually not enough for free fluoride ions to form. Therefore, compared to some other substances, sodium monofluorophosphate is less effective.
Sodium fluoride is known for its remineralizing properties. When using this compound, free ions are generated very quickly, which guarantees the effectiveness of the substance.
The remineralization quality of amino fluoride is even better. This compound generates a film on the tooth surface that supplies fluoride to the tooth for a long time, which ensures tissue strengthening. Toothpastes containing this substance are considered the most effective and efficient preventive agents for preventing caries.
Clinical researches
Clinical studies have proven that regular use of professional toothpaste ASEPTA REMINERALIZATION improved the condition of the enamel by 64% and reduced tooth sensitivity by 66% after just 4 weeks.
Sources:
- Report on the determination/confirmation of the preventive properties of personal oral hygiene products “ASEPTA PLUS” Remineralization doctor-researcher A.A. Leontyev, head Department of Preventive Dentistry, Doctor of Medical Sciences, Professor S.B. Ulitovsky First St. Petersburg State Medical University named after. acad. I.P. Pavlova, Department of Preventive Dentistry
- Study of the clinical effectiveness of treatment and prophylactic agents of the Asepta line in the treatment of inflammatory periodontal diseases (A.I. Grudyanov, I.Yu. Aleksandrovskaya, V.Yu. Korzunina) A.I. GRUDYANOV, Doctor of Medical Sciences, Prof., Head of Department I.Yu. ALEXANDROVSKAYA, Ph.D. V.Yu. KORZUNINA, asp. Department of Periodontology, Central Research Institute of Dentistry and Maxillofacial Surgery, Rosmedtekhnologii, Moscow
- Clinical studies of antisensitive toothpaste “Asepta Sensitive” (A.A. Leontyev, O.V. Kalinina, S.B. Ulitovsky) A.A. LEONTIEV, dentist O.V. KALININA, dentist S.B. ULITOVSKY, Doctor of Medical Sciences, Prof. Department of Therapeutic Dentistry, St. Petersburg State Medical University named after. acad. I.P. Pavlova
Connection Features
Currently, in industry, sodium monofluorophosphate is mainly used for the production of toothpastes. In this case, an active free ion is generated during the hydrolysis reaction that occurs when brushing teeth. The more acidic the environment, the faster this chemical reaction will occur.
Pastes containing sodium monofluorophosphate are especially actively advertised both by manufacturers and as part of social programs aimed at improving public health. It is not difficult to use such therapeutic and preventive products for cleaning the oral cavity, their price is no different from other products, and the benefits are obvious.
For industrial use, sodium monofluorophosphate is obtained by treating silver, ammonium monofluorophosphates or neutralizing monofluorophosphoric acid. It is noted that the industry needs to refine the methods for producing the chemical, since all the methods available today do not produce an insufficiently pure product.
What are the dangers of fluoride deficiency and excess?
Low fluoride content weakens tooth enamel, making it more susceptible to tooth decay. But excess fluoride is more dangerous than its deficiency. The teeth and bones are the first to suffer - they become fragile. Metabolic disorders, blood clotting and other serious health problems can also be observed.
So, at the moment, science has not proven the harm of fluoride-containing toothpastes, but there are many facts indicating their benefits. Which toothpaste should you choose for quality dental care? Your dentist will recommend the best option based on the current clinical situation, your complaints and wishes.