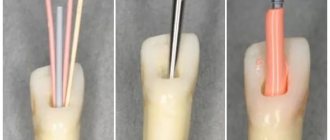
Giuseppe Cantatore University of Verona (Italy). Department of Endodontics. Assistant professor.
In a classic study published in 1985, Bystrom et al. compared the sterilizing effectiveness of three different methods of endodontic treatment of infected canals and found that mechanical treatment in combination with irrigation with saline solution ensures canal sterility in 20% of cases, while replacing NaCl with a 5% sodium hypochlorite solution leads to canal sterility in 50% of cases. cases, and the addition of the latter scheme with a single temporary filling of the canal with calcium hydroxide increases the percentage of canal sterilization to 97%. Does this mean that when treating infected root canals, temporary filling with medicated paste is required in all cases? More than 15 years have passed since the Bystrom et Al study; Today we know much more about the properties of microorganisms associated with pulp-periodontal pathology: from virulence to mobility, from the ability to penetrate dentinal tubules to sensitivity to various antiseptics. 15 years of research experience have shown that many irrigation solutions have pronounced bactericidal activity against microorganisms such as enterococcus faecalis or candida, which are resistant to calcium hydroxide or chlorophenol. In this article, we will discuss in 7 points how to improve the root canal irrigation procedure, using the right products in the right sequence, in order to reduce the need for additional medicinal treatment of the canals between visits.
Key Factors for Effective Cleaning and Irrigation of the Root Canal System
1. Careful diagnosis of existing pulp-periodontal pathology
2. Taking into account the condition of the tooth tissues and the complexity of the anatomy of the root canal system 3. Removal of the endodontic smear layer 4. Compliance with indications when choosing means for irrigation 5. Optimization of the active components of the irrigation solution 6. Correct sequence of application of the irrigation solution during root canal treatment 7. Mandatory costs of at least 5 minutes for irrigation before filling
Myths and legends of endodontics. EDTA.
When you communicate with colleagues, whether in person, online or at lectures, you very often come across questions and opinions about EDTA, ranging from recommendations to the most ridiculous speculations and myths. I would like to first list them, and then proceed to a detailed analysis of each of them “point by point”.
So:
1. EDTA in dentistry is an acid; it can be replaced by other weak acids - citric, maleic, etc.
2. EDTA does not have antiseptic effects - its use is not necessary.
3. EDTA helps to locate root canal orifices. A tampon with EDTA can be left in place for a day (two, three) if the canals cannot be detected.
4. EDTA-based gels soften dentin and facilitate the work of instruments in the canal; they are used as lubricants so as not to break the instrument.
5. EDTA removes the smear layer.
By popularity, it would be necessary to distribute the points in reverse order, but then it would be more difficult to describe, jumping from beginning to end and vice versa.
1. EDTA in dentistry is primarily a neutral salt.
Initially, ethylene diamine tetraacetic acid (EDTA) is a tetrabasic acid with four acetate acid groups. But it is not used in the form of acid due to the extremely low concentration of the saturated solution, only 0.02%.
As you know, endodontic preparations have a concentration of 17%; a pure acid solution cannot create such a solution. This is why its salts are used. Ethylene diamine tetra acetates (EDTA). The dissolution of the mineral components of dentin by these salts is due not to acidic interactions, but to complex formation. As a result, a multivalent metal ion is embedded inside a salt molecule, being drawn into it by 6 ionic bonds. In this case, acid radicals do not participate in the mechanism. That is, an EDTA salt with any substitution is capable of removing calcium from dentin hydroxyapatite - be it sodium salt, disodium or tetrasodium salts.
If you look at the solubility table:
It becomes clear that brands of dental drugs use trisodium-tetrasodium EDTA or a mixture of both.
All of them have the same complexing activity and neutral (with a shift to alkaline) pH adjustment.
From now on we will call them one abbreviation – EDTA.
The described compound has found wide application in industry - it is used for the isolation of metal ions - for example, for the isolation of uranium from uranium ores (EDTA uranates are the only insoluble complex).
In analytical chemistry - the so-called complexometry of salts.
In the cleaning activities of housing and communal services - for cleaning boilers and pipes from scale and metal oxides.
In medicine and conservation - as an ampoule preservative for many drugs (binds metal ions released from glass ampoules)
In toxicology - administered intravenously as antidotes for poisoning with heavy metals and radionuclides.
And nowhere is EDTA used as an acid, only in the form of salts. Due to the neutral reaction of the environment and the ability to remove polyvalent metal ions from any strong salt or oxide (for example, EDTA removes iron from any oxides - Fe2O3, FeO2 - rust), but does not interact with pure metals, in many situations, including endodontics, replace with weak ones it is impossible with acids.
EDTA still has an antiseptic effect, albeit a very limited one.
But unique. Due to the ability to remove calcium from almost any structure containing it, EDTA can also remove calcium from chitin, the main component of the cell wall of fungi. Therefore, it has a pronounced fungicidal effect against Candida albicans and Aspirgillus nigrum, which are often endopathogens. In relation to the rest of the microflora, they do not show any antiseptic effect.
Preliminary conclusions from what has been said may be as follows: EDTA solutions can be successfully used for chelate demineralization of canal walls, debris, and smear layer elements. For treatment of the canal, in case of suspicion of the presence of glandular flora (the tooth was open), without fear of a cytotoxic effect, in case of EDTA getting into the perapical tissues or onto the gingival mucosa.
EDTA cannot help identify root canal orifices. For many reasons.
Firstly, often the mouths of the canals, in vital and non-vital cases, are covered with organic components of living or necrotic pulp, which is an insurmountable obstacle for EDTA. In complex cases of calcification of the coronal cavity of the tooth, the orifices are closed by massive dentin peaks. EDTA, regardless of the concentration, after an exposure of 5 minutes destroys the mineralized layer of dentin by only 20-30 micrometers, an exposure of 30 minutes - 30-40 micrometers, an exposure of 24-48 hours - 50 micrometers, further progression does not occur due to the saturation of the chelate. That is, a tampon with EDTA left for a day will “eat” only 0.05 mm of dentin, which certainly will not contribute to the visual or tactile identification of the root canal orifices.
Most often, such a statement is due to the freshness of the doctor’s view of the situation of the bottom of the tooth cavity, after a day - two. After two days, we already forget how and by what stereotype or landmark we looked for the entrance to the canal last time, and we begin to search a little differently, unexpectedly for ourselves, finding what we were looking for. EDTA has nothing to do with it, it is quickly used up and after a while it self-limites - it stops working. Therefore, you can find many practitioners who claim that this technique works, but do not give an account of why. They ultimately attribute this to the “miraculous opening of the EDTA mouths.”
EDTA-based gels make the instruments easier to work with. But only manual ones.
The fact is that the feather gel on EDTA was Rc-prep, which had the composition (and indeed has) - 15% EDTA, 10% carbamide peroxide, polyethylene glycol (gelling agent). It was introduced in 1968 to facilitate canal work with HAND tools.
It also works by chelating the surface of the canal wall (softening a layer of 1-2 microns), making it easier to work with during manual filing.
It’s not for nothing that I highlighted the word “manual”. The rotation speed of a hand tool is on average half a revolution per few seconds. Very low. We work with finger files extremely slowly, and the chelation softening process is very slow. The EDTA from the gels manages to act on the wall and soften it while we are twisting there.
Now imagine that the speed of file processing has increased a hundred times. For example, up to 300 rpm. We moved to mechanical nickel titanium instruments. The speed of wall processing by the edges of a rotary tool exceeds the rate of calcium chelation with EDTA. As a result, chips become embedded in the thick gel between the blades of the tool, sharply increasing the torsion load on the file.
First, we noticed a sharp increase in torsional load when using gels. Then we just studied it. It turned out that according to the laws of hydraulics, in the size of the root canals and the speed of operation of rotary instruments (length 10-15 mm, diameter 0.2-0.4 mm, rotation of the cutting edge 250 rpm and higher), any liquid provides greater lubrication than any gel, regardless of chemical formula.
Any rotary nickel-titanium instruments in a viscous gel environment collect sawdust on the verge faster and more dangerously than in any liquid, even if it does not have chelating properties. For example, in a hypochloride solution.
In addition, there is a negative point - tools made of Ni-Ti alloys have shape memory, and due to the inability to maintain bending, they always tend to straighten. Every time we treat the canal with EDTA and then pass it with a rotary instrument, we force the instrument to cut the dentin more aggressively, provoking transport of the canal.
Intermediate output. Working with rotating nickel-titanium tools with EDTA rinsing or adding it in the form of a gel does not improve sliding, but rather increases clogging of the edges and can lead to torsional overload or failure. At the same time, increasing the risk of transportation, straightening, and even strip perforations in the canal.
And as a completely separate point, I would like to highlight the most common misconception, which sounds like “EDTA removes the smear layer”
This is not even a misconception, but simply a distortion of the truth. EDTA HELPS to remove the smear layer.
Let’s first figure out what this smear layer is.
The smear layer is a conventional name in enodontology, denoting a surface that has been subjected to mechanical processing, and on which, as a result of tool friction, a special structure has formed from the products of friction/cutting dentin.
Let us remember the structure of the smear layer - it consists of two (and according to other data, three (!) sublayers). The surface is the actual smear layer, on the surface and in the thickness it consists of broken hydroxyapatite crystals, slightly deeper mineralized crystals are mixed with dentin collagen (vital case) and biofilm elements, dead and living microorganisms. Under the autumn layer lie the so-called “smeared plugs” - only mineralized components of fragments of hydroxyapatite crystals.
Conventionally, the smear layer consists of:
1. Mineralized
2. Organic
3. Re-mineralized
Layers, each of which is 1-2 nanometers thick. EDTA can only dissolve the mineralized component, provided that each layer is a barrier. That is, by acting on the chelating agent EDTA, we dissolve only the surface mineralized part of the smear layer; the remaining organic matter is a barrier for EDTA.
Conclusion - to remove the smear layer, we need two substances - one that removes mineralized hydroxyapatite and one that dissolves organic matter of any kind. We come to the conclusion that to remove the smear layer we need a whole sequence of exposure to two reagents - first EDTA (remove the mineralization of the upper part), then sodium hypochloride (remove torn collagen, remnants of pulp, microbes, bioplastic matrix), and only the mineralized layer of smeared plugs remains in the tubes - EDTA again.
This way the EDTA does not remove the smear layer. If you worked with tools in hypochloride and at the end washed it with EDTA, thinking that you had removed this formation, I will disappoint you. Unfortunately no. EDTA is a CRITICAL COMPONENT OF THE smear layer REMOVAL PROCEDURE, but without a specific sequence, it is powerless alone.
You can often hear that “I use EDTA (Rc-prep, rc-cream, glyde) in the processing process - a smear layer DOES NOT FORM.” I’m sorry to say, but the formation of a smear layer structure is the result of the physics of friction and does not depend on the presence or absence of chelates or lubricants. Even if the presence of EDTA at the time of cutting dentin with a machine tool (and this is contraindicated and unsafe) is present in the canal, it cannot in any way remove the organic component of collagen and microbes, and there will be plugs under them.
That is, a smear layer, even with the use of gels or liquid EDTA during instrumentation, is formed regardless. Always.
There is one more significant point. In the process of any instrumental processing, not only a smear layer of dentin is formed, but also an abundant accumulation of dentin filings, debris and a mixture of organic matter in the anatomically difficult parts of any canal.
I think it is known that 80% of the canals are not round in shape, and 60% of them are long oval - that is, they do not have a round configuration up to the apex. We’ll talk about clogging sawdust with various systems of various anatomically complex tooth formations next time.
In the meantime, about the sawdust in the isthmus and fins.
The fact is that only two methods can knock out sawdust pressed with a rotary instrument into anatomical complexities - ultrasound-activated irrigation (until 2015 it was called PUI - Passive Ultrasonic Irrigation. In 15th, the International Endo Society decided to change the term. Now it is UAI - Ultrasonic Irrigation activated irrigation), and the second technique is sonic activation of hypochloride with intermittent irrigation of EDTA.
That is, the procedure is the same as in the case of a smear layer - EDTA - HYPOCHLORIDE - EDTA - HYPOCHLORIDE. Hypochloride is activated, EDTA is not. This makes it possible to clean debris and sawdust in areas where ultrasound cannot reach - for example, behind a bend, in the area beyond the first bend of a double bend, that is, in most curved canals or canals of teeth with complex anatomy.
A serious conclusion can be drawn from this, burdened by recommendations regarding the smear layer.
So. When we WILL remove the smear layer.
1 Work in infected canals in one visit.
If we want to wash the infected canal with irrigants (this is not difficult - an anatomical canal, without hidden anatomy) and seal it at the same visit. Once we have completed the instrumentation, we will do a smear layer removal procedure (EDTA-Hypochloride-EDTA), after which we will clean the canal with different irrigation methods. The point is to open all the isthmus, remove the clogging of sawdust, open the dentinal tubules, so that it can all be washed as much as possible with hypochloride and sealed as tightly as possible. That is, we remove the smear layer before the active phase of irrigation.
Work in infected canals in two visits with the introduction of calcium hydroxide for 2-3 weeks.
We remove the smear layer at the end of the first visit, after instrumentation, BEFORE applying calcium hydroxide. Our goal is to free up the entire irregular anatomy of the dentinal tubules as much as possible, so that the increase in pH from calcium hydroxide is as rapid as possible. Removal of the smear layer will accelerate the action of the hydroxide, and is improved by increasing the rate of increase in the alkalinity of the environment in dentin.
4 Work in uninfected canals in two visits, with the addition of calcium hydroxide.
Yes, this point will surprise many. They say, if the primary case is uninfected, why is there hydroxide, we can do everything at once... I’m sorry, but experience gives us rare cases when we can’t. The simplest thing is a complex anatomical seashape. You have processed everything, but you have doubts. And after careful processing, he gives you a surprise in the form of another apex channel.. And everything has been processed, but in this branch there is still nothing.. the profile did not spin.. And time is running out.. Process the seashape, remove the smear layer, put in calcium hydroxide and leave it even for a week - that is, until the calcium has an antiseptic effect (2-3 weeks), but has already dissolved sterile organic matter in the lumen. To ensure excellent dissolution and organolytic work of calcium, remove the smear layer before adding it according to the scheme - EDTA, Hypochloride, EDTA.
Vital cases that we treat in one visit but with presumably complex anatomy.
Here it would be necessary to make a lyrical digression to research on the area of contact of the instruments, and on what we are achieving. If we want the canal to be as disinfected as possible, and in vital cases hypochloride is needed mainly to burn organic matter so that it does not become a medium for microbial growth in the future... then we need to remove even the non-smeared layer from the surface of the dentin - in this case we need it didn't go into it.
We need to dissolve and remove debris plugs in canals of complex anatomy. From isthmuses, isthmuses, fins... Those volumes where the untouched pulp will remain, covered with sawdust from the all-dissolving action of Hypochloride.
By using the smear layer procedure, we can improve irrigation and prognosis in uninfected cases of complex anatomy.
Vital cases with simple anatomy.
We absolutely DO NOT REMOVE the smear layer. These are single-canal or double-canal teeth with non-corrupted canals, large straight canals, etc. Where there is no statistical probability of manifestations of complex or atypical anatomy. In simple, smooth, uncomplicated canals, the smear layer will, on the contrary, act as a barrier in case of operational errors and accidental infection of the canals. But still, this will not save you from an open tooth.
Indications for use
- heavy metal poisoning;
- angina pectoris , atherosclerosis , arrhythmias , hypertension, circulatory disorders in the extremities;
- increased levels of cholesterol, triglycerides, C-reactive protein, glucose, blood clotting;
- diabetic neuropathy;
- chronic fatigue syndrome;
- insomnia , memory impairment, irritability, depression, age-related changes in intelligence;
- multiple sclerosis , and Alzheimer's disease ;
- bursitis , osteoarthritis ;
- cataract , glaucoma ;
- psoriasis;
- allergic diseases;
- frequent ARVI;
- impotence , prostatitis .
Environmental Consequences
Abiotic degradation
EDT is in such widespread use that questions have been raised whether it is a persistent organic pollutant. While EDTA serves many positive functions in a variety of industrial, pharmaceutical and other applications, EDTA's longevity can create serious problems in the environment. EDTA degrades slowly. It occurs mainly abiotically in the presence of sunlight.
The most important process for the elimination of EDTA from surface waters is direct photolysis at wavelengths below 400 nm. Depending on light conditions, the photolysis half-life of iron(III)EDTA in surface waters can range from as low as 11.3 minutes to over 100 hours. Degradation of FeEDTA, but not EDTA itself, produces iron complexes of triacetate (ED3A), diacetate (EDDA), and monoacetate (EDMA) - 92% of EDDA and EDMA decomposes in 20 hours with ED3A showing significantly higher resistance. Many environmentally abundant types of EDTA (eg Mg 2+ and Ca 2+ ) are more persistent.
biological decay
In many industrial water treatment plants, EDT elimination can be achieved at approximately 80% using microorganisms. The resulting byproducts are ED3A and iminodiacetic acid (IDA)—suggesting that both the backbone and acetyl groups are attacked. Some microorganisms have even been found to form nitrates from EDTA, but degrade optimally under moderately alkaline conditions pH 9.0-9.5.
Several bacterial strains isolated from wastewater treatment plants effectively degrade EDTA. Specific strains include Agrobacterium radiobacter
ATCC 55002 and sub-clades of Proteobacteria like BNC1, BNC2 and strain DSM 9103. The three strains have similar aerobic respiration properties and are classified as Gram-negative bacteria. Unlike photolysis, the chelated species is not exclusively iron(III) so as to be degraded. Rather, each strain uniquely consumes varying metal-EDTA complexes through multiple enzymatic pathways. Agrobacterium radiobacter only degrades Fe(III), while EDTA BNC1 and DSM 9103 are unable to degrade iron(III)EDTA and are more suitable for calcium, barium, magnesium and manganese(II) complexes. EDTA complexes require dissociation before degradation.
Formula, release form
The formula of EDTA is C10H16N2O8.
Main forms of release:
- Powder packaged in 10 grams.
- Solution in ampoules of 20 milliliters, 10 pieces per package.
- In capsules, 30 pieces per package. One capsule can have different dosages: 625, 440, 500 milligrams.
The cost of a pure product varies from 200 to 1000 rubles, depending on:
- cities of purchase;
- networks of pharmacies where acid is sold;
- manufacturer's name;
- forms of purchase and dosage.
EDTA is available without a prescription. The shelf life is 2 years from the date of manufacture. This date can be found on the top of the package.
This shelf life is observed if the drug was properly stored at a temperature not exceeding 25 degrees.
pharmachologic effect
It exhibits the property of an antidote when cyanogen chloride, hydrocyanic acid or volatile cyanide compounds enter the body. In addition, activity is observed against the following toxic substances:
- mercury;
- lead;
- zinc;
- arsenic;
- cobalt.
It has the ability to bind toxic particles in the bloodstream and then remove them along with urine and feces.
Being an antioxidant, it prevents oxidation processes. Other useful properties include:
- stimulation of immunity;
- normalization of cholesterol and glucose levels;
- blood thinning;
- restoration of physiological, biochemical processes in the internal systems of the body.
In pharmaceuticals, it is added to the composition of many drugs in order to enhance the therapeutic properties of the latter and increase the bioavailability of the active components.
Principles of coordination chemistry
Metal-EDTA chelate as found in Co(III) complexes.
The structure shows that the EDTA 4-ligand does not completely encapsulate Fe(III), which is seven-coordinate.
In coordination chemistry, EDTA 4- is a member of the aminopolycarboxylic acid family of ligands. EDTA 4- usually binds to the metal cation through its two amines and four carboxylates. Many of the resulting coordination compounds adopt octahedral geometries. Although of particular importance for its application, these octahedral complexes are chiral. Cobalt(III) anion has been resolved into enantiomers. Many EDTA 4- complexes take on more complex structures, resulting either in the formation of additional bonds with water, i.e.
seven coordinates of the complexes, or the displacement of one carboxylate arm of water. Iron (III) complex EDTA seven-coordinate. Early work on the development of EDTA was carried out by Herold Schwarzenbach in the 1940s. EDTA forms particularly strong complexes with Mn (II), Cu (II), Fe (III), Pb (II) and Co (III).
Several features of EDTA complexes are relevant to its applications. Firstly, due to its high dentacy, this ligand has a high affinity for metal cations:
3+ + H 4 EDTA ⇌ — + 6 H 2 O + 4 H +
K
eq = 10 25.1
Written in this way, the equilibrium factor indicates that metal ions compete with protons for binding to EDTA. Since metal ions are widely covered by EDT, their catalytic properties are often suppressed. Finally, since EDTA 4- complexes are anionic, they tend to be very soluble in water. For this reason, EDTA is capable of dissolving deposits of metal oxides and carbonates.
V p K
and the values of free EDTA are 0, 1.5, 2, 2.66 (deprotonation from four carboxyl groups) and 6.16, 10.24 (deprotonation from two amino groups).
Main manufacturers
In Russia, sodium calcium ethylenediaminetetraacetate is produced by the State Unitary Enterprise “Experimental Plant of the Academy of Sciences of the Republic of Belarus” (Bashkortostan). The small volume of production does not allow satisfying the demand for the food additive.
The group offers EDTA food additive from the manufacturer Jan Dekker (Netherlands).
The domestic market is formed by Chinese suppliers:
- Humica Weihai International. The chemical enterprise specializes in the production of antioxidant E 385 and has its own scientific laboratory;
- Hangzhou Anxing Trade, a company operating since 1993, supplies products to 40 countries;
- Shijiazhuang Xinlongwei Chemical, a company part of the Landchem chemical corporation, has international product quality certificates.
Despite the low toxicity of the food additive E 385, it should be treated with caution. The substance has exactly the opposite properties: it can both remove heavy metals from the body and promote their accumulation and absorption into the blood. The effect depends on the amount and duration of use.
Environmental organizations are also sounding the alarm. Once in the surrounding space, EDTA does not break down into simple components, like other chelate compounds. The safety of the ecological system is at risk.
Reviews
Based on the results of these studies, it was concluded that the substance does not pose a threat to the environment and is safe for people and animals. The positive safety finding has expanded the use of this substance, as evidenced by the growth of the EDTA market in recent years.
In the experiments of T.L. Dubina, which were carried out in 1968-1975, the following conclusions were drawn: “... slows down the development of atherosclerosis, hypertension and hypercholesterolemia. Does not affect the aging process, but prolongs life by reducing morbidity."
reviews about chelation therapy with EDTA; most often these are reviews from foreigners, which are submitted in translation. Cheletotherapy is more common abroad - USA, Israel, Canada. Mexico. Reviews are varied.
“I use chelation and my lipids are normal.”
“...took 250 mg. My vision has improved with EDTA."
“My husband has diabetic neuropathy - after taking it, his blood circulation improved.”
“...I feel much better, less pain in joints and muscles.”
“It’s clear if used for heavy metal poisoning, and not for an experiment on a healthy organism.”
"There is a potential for significant impairment of kidney function as bound heavy metals pass through the kidneys."
Synthesis
The compound was first described in 1935 by Ferdinand Münz, who prepared the compound from ethylenediamine and chloroacetic acid. Today, EDTA is primarily synthesized from ethylenediamine (1,2-diaminoethane), formaldehyde, and sodium cyanide. This route yields tetrasodium EDTA, which is converted in a subsequent step to acidic forms:
H 2 NCH 2 CH 2 NH 2 + 4 CH 2 O + 4 NaCN + 4 H 2 O → (SAK 2 CCH 2 ) 2 NCH 2 CH 2 N (CH 2 CO 2 Na) 2 + 4 NH 3 (SAK 2 CCH 2) 2 NCH 2 CH 2 N (CH 2 CO 2 Na) 2 + 4 HCl → (HO 2 CCH 2) 2 NCH 2 CH 2 N (CH 2 CO 2 H) 2 + 4 NaCl
This process is used to produce approximately 80,000 tons of EDTA each year. Impurities produced by this route include glycine and nitrilotriacetic acid; they arise from ammonia coproduct reactions.